Past Issues
In Vitro Establishment, Hydroponic, Salt Tolerance, Antifungal and Phytochemical Investigations in Important Sand Binder Medicinal Plant Ipomoea pes-caprae
Shamshad Ahmad Khan1*, Sara Rashed Salem Al Faraji 1, Mira Said Hamed Almukhaini1, Ayaz Mohd 1, Farrukh Jamal2, Samuel S D Premkumar3 and Priyanka Verma4
1Applied Biotechnology Department, University of Technology and Applied Sciences, Sur, Sultanate of Oman
2Department of Biochemistry, Dr. R.M.L. Awadh University, Ayodhya, India
3Central Analytical & Applied Research Unit, College of Science, Sultan Qaboos University, Sultanate of Oman
4 Ex-pool scientist, CSIR-National Chemical Laboratory, Division of Biochemical Sciences, Pune, Maharashtra, India
*Corresponding author: Shamshad Ahmad Khan, Applied Biotechnology Department, University of Technology and Applied Sciences, Sur, Sultanate of Oman; Email: [email protected]
Received Date: January 20, 2023
Publication Date: February 20, 2023
Citation: Khan SA, et al. (2023). In Vitro Establishment, Hydroponic, Salt Tolerance, Antifungal And Phytochemical Investigations In Important Sand Binder Medicinal Plant Ipomoea pes-caprae. Traditional Medicine. 4(1):14.
Copyright: Khan SA, et al. © (2023).
ABSTRACT
Ipomoea pes-caprae (L.)R. Br. (Beach Morning Glory) is a latex-producing creeper with long stems and deep tap roots characterized by its ability to tolerate salinity and salty air. It has environmental impact as a sand binder. This medicinal plant is known to be astringent, laxative, tonic, alternative, and diuretic in traditional medications. Present study deals with the optimizations of in vitro protocols to raise and multiply plant at different morphogenic levels. It was found that nodal explants were showing new bud generation on MS+ 3.0 mg/L BAP (6-Benzyl amino purine) with generation frequency of 90.00±2. The best rooting response was observed on MS+ 1.0 mg/L IBA (Indole-3-butyric acid) where maximum number of roots (8.0±1.0) were generated with 80% acclimatization rate in lab conditions. Moreover, salt tolerance studies were performed in hydroponic system. In comparison to liquid MS (Murashige and Skoog) basal media and distilled water, hydroponic system was found to be optimum in roots and shoot generation. Out of four concentrations (0.2- 0.8%) of sodium chloride (NaCl) used in present study, 0.4% NaCl salt concentration showed optimum growth of root and shoot simultaneously. In vitro established plants were subjected to FTIR (Fourier transform infrared spectroscopy) and GC-MS (Gas chromatography–mass spectrometry) analysis and were showing array of compounds. The methanolic extract of lab acclimatized plants showed modest antifungal activity against Pythium and Alternaria fungal genus with percent inhibition of 17.71 and 8.03, respectively on 6th day readings.
Keywords: Ipomoea pes-caprae, Shoot multiplication, Hydroponics, Salt tolerance, GC-MS Analysis, Antifungal activity
INTRODUCTION
Known by many common names such as Goat’s foot, Sea Morning Glory and Beach Morning Glory, Ipomoea pes-caprae (L.) R. Br. is potent sand binder plant belonging to the family Convolvulaceae with more than 200 species known of this plant is a trailing, creeping vine [1]. This herbaceous plant is known for its salt tolerant abilities and related salty atmosphere henceforth found abundantly in moderate to high saline areas and coastal regions [2]. In Sultanate of Oman, this plant system is found in the coastal areas of Dhofar governorate and prefers to grow in tropical and sub-tropical areas worldwide. Often found to be covering the area because of its growth this perennial plant has lengthy stems. The I. pes-caprae plant is a sand stabilizing plant and can easily make a rapid growth in the dune areas especially in the seaward slope zones. This plant has a common use as an aboriginal medicine for treating the stings of sting ray and stone fish [3]. In many other countries this plant is used to treat gastrointestinal ailments, inflammations, auto immune diseases, piles, odema and colic diseases. The phytochemical investigations in this plant have revealed that methanolic extract from floral segments has anti-inflammatory, hemolytic and antimicrobial properties [4]. The anti- nociceptive activities of the methanolic extract have also exhibited effectiveness in relieving pain in mouse models [5]. The antifungal and antimicrobial activities of the plant extract were also considerable against those fungal and bacterial strains which are known to causes infections in human beings [4]. The chemical analysis of the I. pes-caprae further have indicated the presence of alkaloids, flavonoids, terpenoids and steroids [6]. The I. pes-caprae phytochemical study led to characterization of a range of compounds such as betulinic acid, glochidone, isoquercitrin, alpha- and beta-amyrin acetate etc. [7]. Apart from these compounds additional compounds such as indolizidine alkaloids, ergoline alkaloids, nortropane alkaloids, coumarins, norisoprenoids, phenolic compounds, diterpenes, benzenoids, flavonoids, isocoumarins, triterpenes and anthocyanins have also been reported from this genus of medicinal plant [8]. Recently the therapeutic and nutritional activities of I. pes-caprae have been validated as its extracts have been known as a collagenase inhibition agent with the added advantage of not causing any cytotoxic effect. It is a well-known fact that damage of collagen in skin dermis causes wrinkle and skin damage hence this plant system can play an important role in developing products related to skin care. The recent study found that extract of I. pes-caprae at a concentration of non-cytotoxic levels may help in enhancing proliferation of cells, production of collagen and wound healing abilities henceforth there is a positive indication that these extracts can be used in cosmeceutical industry to be used as a potential anti-aging agent of skin [9].
Plant productivity and growth are severely affected by soil salinity as high level of salt concentrations with shortage of water can be a lethal combination in causing imbalance in ionic conditions, causing extensive osmotic stress which may result in secondary stresses to the soil and plant as well [10]. The salt loving plants such as I. pes-caprae are difficult to grow under in vitro conditions because of mainly two reasons: (i) due to their heterozygotic nature (ii) and the complications involved in raising these kinds of plants under in vitro conditions [11]. Though it's very important to address these problems associated with halophytic plants as such plants may work as ideal model systems to assess the adaptative mechanism they involve to deter the high salinization conditions. The in vitro studies related to saline conditions in this plant may also help in stabilizing these plants under high salinity zones where they may work effectively as a soil binder to stop the threating soil erosion problem in future.
Hydroponics is a technique of gardening or cultivating plants in the absence of soil, wherein water provides hydration, oxygen and nutrients to plant life. This type of agricultural system can use minimal spaces, less water with percentage less than 90 than conventional agriculture where invented design may help in plant growth in shorter time. Hydroponic systems act by permitting accurate control in the environmental conditions such as pH and temperature balance and increased exposure to water and several nutrients. Hydroponic culture has several benefits such that any plant can grow rapidly with very less amount of water rather than those consumed in conventional cultivation. In addition, plants have the ability to grow in more sterilized environment without using pesticides. Also, hydroponic medium consumes very less space for cultivation and preserves more amount of water. Moreover, nutrients balance can be controlled; harvesting process is easier and no need of tilling, mulching, weeding and changing of the soil is required [12].
The in vitro establishment studies in I. pes-caprae have not been fully explored as scarcely reports of tissue culture from this plant is available. For faster multiplication in I. pes-caprae, media combinations have been optimized. Previously with MS supplemented with zeatin was found to be effective to regenerate shoots from nodal explants but no axillary branching was noted [5]. Henceforth a detailed in vitro insight is attempted in present study for optimization of a better multiplication media for rapid growth, callus induction, rooting and acclimatization of plants under greenhouse conditions. Experiments were also conducted to optimize hydroponic system for simultaneous root and shoot growth with salt supplementation. Phytochemical analysis and antifungal activity of lab acclimatized plants was also conducted and discussed in this study.
MATERIALS AND METHOD
Plant Collection and Sterilization
Explants source (leaves and nodes) were taken freshly from the Ipomea plant from a registered Nursery in Sur, Oman and thorough washing was done with running tap water followed by detergent (Tween 20) washing. After the thorough washing and removal of sap from cut ends, treatment of one minute with ethanol was employed. The surface sterilization steps for nodal and leaf explants were conducted in the sterilized conditions in a laminar flow by using 0.1 % mercuric chloride. It was followed by four times washing with autoclaved distilled water. Further inoculation of explants in sterilized media was performed on the laminar flow.
Shoot Multiplication
The freshly cut and sterilized nodal explants were shifted to MS media [13] supplemented with range of 6-Benzyl amino purine (BAP) combinations mentioned in the Table1. Data collection was conducted after the completion of 6 weeks and a mean performance of four replications for each of the treatment was considered. The morphogenic changes were noted in form of mean percentage of explants which responded and the shoot bud number induced from each of the responsive explants. The well-established plants were subjected to rooting followed by shifting to glass house conditions for further establishment and phytochemical analysis studies.
Table 1: Morphogenic response of I. pes-caprae explants on different media compositions.
|
Purpose |
Media composition |
Type of response (Four weeks) |
Frequency of regeneration (%) |
No. of shoots/ extent of callus (+) /roots per explant |
Complete rooted plants recovery (%) |
Green house acclimatization (%) |
|
Shoot bud initiation and multiplication. |
MS+ 1.0 mg/L BAP |
Shoot bud initiation but no further growth. |
45.00±1.2 |
|||
|
MS+ 2.0 mg/L BAP |
Shoot bud initiation but no further growth. |
50.00±1.2 |
||||
|
MS+ 3.0 mg/L BAP |
Shoot bud initiation and shoot multiplication on transfer to the same medium composition. |
90.00±2.2 |
4.00±1.0 |
90.00±1.0 |
||
|
MS+ 4.0 mg/L BAP |
Node swelling but no growth. |
- |
||||
|
MS+ 5.0 mg/L BAP |
Node swelling but no growth. |
- |
||||
|
MS+ 6.0 mg/L BAP |
Node swelling and callusing |
40.00±2.0 |
++ |
|||
|
MS+ 7.0 mg/L BAP |
Node swelling and callusing |
60.00±1.0 |
+++ |
|||
|
Multiplication of callus obtained from nodal segments |
MS+ 1.0 mg/L BAP+1.0 mg/L NAA |
Browning of the explants |
- |
|||
|
MS+ 2.0 mg/L BAP+1.0 mg/L NAA |
Browning of the explants |
- |
||||
|
MS+ 3.0 mg/L BAP+1.0 mg/L NAA |
Callus proliferation |
30.00±3.0 |
+ |
|||
|
MS+ 4.0 mg/L BAP+1.0 mg/L NAA |
Callus proliferation |
50.00±2.0 |
++ |
|||
|
MS+ 1.0 mg/L BAP+2.0 mg/L NAA |
Callus became green and hard without further growth |
- |
||||
|
MS+ 1.0 mg/L BAP+3.0 mg/L NAA |
Greening of explant without further growth |
- |
||||
|
MS+ 1.0 mg/L BAP+4.0 mg/L NAA |
Greening of explant without further growth |
- |
||||
|
MS+ 1.0 mg/L BAP+5.0 mg/L NAA |
Explant blackening |
- |
||||
|
Rooting from in vitro established plants. |
MS+1.0 mg/L IBA |
swelling and by regeneration of thin branched roots |
90.00±1.0 |
8.00±1.0 |
90.00±2.0 |
80 |
|
MS+2.0mg/L IBA |
swelling with regeneration of thick roots |
30.00±1.0 |
5.00±1.0 |
84.00±1.0 |
70 |
|
|
MS+3.0 mg/L IBA |
swelling with regeneration of thick roots |
30.00±2.0 |
5.00±1.0 |
80.00±1.0 |
75 |
Callus Induction
Apart from the shoot multiplication, attempts were made to regenerate callus from freshly sterilized leaf explants. The callus obtained during the shoot multiplication optimization was also tested on various media combinations for further proliferation. The explants were placed on MS media fortified with BAP and NAA (naphthalene acetic acid) in 90mm petriplates (Table1) and were observed for any swelling or callus induction on weekly basis.
In vitro Rooting and Glass House Hardening of Plants
The fully grown shoots were also assessed for their rooting response on MS media supplemented with IBA which ranged from 1.0mg/L to 3.0 mg/L. The rooting response was noted from 7th day up till 28th day on weekly basis. The four weeks old rooted plants were washed with running tap water to remove the agar followed by shifting to a mixture of peatmoss and sterilized soil to in 1:1 combination. To maintain the humidity level the plants were kept initially covered with glass bottles to maintain humidity, for seven days.
Salt tolerance and hydroponic studies
A basic study was conducted to assess the salt tolerant properties of plant I. pes-caprae. The salt tolerance studies were performed in three liquids namely distilled water, distilled water fortified with MS salts and hydroponic solutions. The sodium chloride (NaCl) concentrations ranging from 0.2% to 0.8% were used for the study with controls. Nodal explants were cut from glasshouse grown native plant and washed thoroughly before placing into the respective solutions. The observations were documented successively from 3rd day up to 27th day.
Antifungal studies
The antifungal assay was carried out against two fungal genus namely Alternaria and Pythium and 1.0 mg/mL concentration of plant leaf extract was used which was dissolved in methanol. The potato dextrose agar (PDA; Blulux Pvt Ltd. India) was plated in 90 mm plates and four grooves were created, one in the center and three at the periphery at equal distances. The fungal culture against which the antagonistic activity was to be tested was inoculated on peripheral grooves while 200ul plant extract was added into the middle groove. The PDA plates containing the fungal cultures only and methanol separately were taken as controls. All the plates were incubated at 28±2 ⁰ C. The experiments were set in triplicates and antagonistic activity was measured by calculating the inhibition zones and observations were taken after 3rd and 6th day successively.
Phytochemical analysis of the plants
The dried leaves (1.0 gm) of the lab established plants were used for extraction and leaves were crushed with the help of pestle and mortar followed by extraction with methanol for 12 h. The final sample was dried and made ready with 1.0 mg/ml concentration in methanol. Whole of the process was conducted at the room temperature and repeated thrice. The methanolic extract was filtered through Whatman filter paper grade 1 and filtrate was completely dried using 60 °C temperature. The dried extract was used for the UV, Fourier transform infrared spectroscopy FTIR-ATR (Bruker) and GC-MS studies. For FTIR analysis, (Model: FT-IR Spectrometer ALPHA II Platinum ATR) was used and injection sample 10.0 μl. For GC-MS analysis, the column used was DB5 MS with an injection volume of 1µl and run time of 60 min. The GC model no. was Agilent Technologies 7890B Gc system and MS model no. was Agilent Technologies 5977A MSD.
RESULTS
In vitro Establishment of I. pes-carpae at Various Morphogenic Levels
A total of eighteen different media compositions were assessed with different plant growth hormones namely, BAP, NAA and IBA to establish the I. pes-caprae plants at different morphogenic level under in vitro conditions. The BAP concentration used for shoot bud induction was ranging from 1.0 mg/L to 7.0 mg/L. It was found that lower concentration of plant growth hormone favors shoot bud induction from nodal explants while higher range (6.0-7.0 mg/L) induced callus formation. The optimum axillary shoot proliferation from nodal explant was achieved with MS+ 3.0 mg/L BAP (90.00±2.2) and interestingly plants showed better response in the same combination for multiplication also. Hence the MS+ 3.0 mg/L BAP was selected as the multiplication media among the tested hormonal combinations as the shoot numbers/explant was optimum (4.00±1.0) and recovery of whole plant (90.00±1.0) on this combination was also found to be maximum. (Table 1; Figure 1 (A-F)).
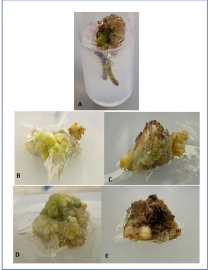
Figure 1: In vitro shoot establishment, rooting and glasshouse acclimatization of I. pes-caprae. (A)-(E) in vitro established shoots on MS+ 3.0 mg/L BAP; (G)-(K) rooting induced by MS+ 1.0 mg/L IBA; (L) well rooted plant ready for transfer to glasshouse conditions and (M)-(O) glasshouse established shoots after 35th day of transfer.
Callus induction was assessed from two explants. Initially sterilized leaf explants were cut and plated on various callusing media combinations of BAP and NAA (Table1). In this case, only swelling of explants was observed and in later stages the explants turned black. This may be due to the hinderance caused by lot of mucilaginous sap inside the leaf which oozes out from fresh cuts. Other explant which was used is nodal segment wherein it was observed that nodal explants showed callusing on higher BAP conc. (Table 1; Figure 2 (A-E)). The induced callus was cut and placed on callusing media combinations. It was found that optimum proliferation frequency (50.00±2.0) was observed on MS+ 4.0 mg/L BAP+1.0 mg/L NAA followed by MS+ 3.0 mg/L BAP+1.0 mg/L NAA (30.00±3.0). In a previous conducted study also, different strength MS media with supplementation of coconut water were achieved successfully for callus induction studies in I. pes-caprae. [11]
Figure 2: Callus induction and multiplication from the nodal segments of I. pes-caprae. (A) callus induction from nodal explants obtained on MS + 7.0 mg/L BAP; (B)-(E) callus multiplication on MS+ 4.0 mg/L BAP+ 1.0 mg/L NAA.
For the root induction studies, the rooting response was noted up till 28th day on weekly basis in shoots placed in multiplication media. But apart from trivial response, no clear rooting was noted in the newly generated shoots of under in vitro conditions. Further the shoots were shifted to 1.0 -3.0 mg/L IBA (Table 1). MS media with 1.0 mg/mL IBA was found to be best suited for rooting in I. pes- caprae shoots, as the regenerated roots were thicker, longer and more in number (8.0±1.0) as compared to the other tested combinations. The frequency of root generation (90%±1.0) was also better compared to other hormonal combinations tested (Table 1; Figure1). The rooted plants were acclimatized in different peatmoss and sterilized soil combinations in lab conditions (28±4 °C temperature and 60–70 % relative humidity) and it was found that 1:1 combination of peatmoss and soil was better for acclimatization as survival rate was up to 90% on this combination.
Hydroponic establishment and salt tolerance study of I. pes-caprae plants
For hydroponic studies, three liquids (hydroponic solution (HP), distilled water with MS salts (DWMS) and distilled water (DW) were taken and response was noted on the basis of simultaneous growth of shoots and roots. It was found that after ten days, the shoots placed in HP and DW were green and growing, while shoots placed in DWMS were showing leaf yellowing (Figure 3A-C). Similarly, on 27th day, optimum plant growth with roots regeneration was observed in HP followed by DW and DWMS (Figure 3D-F).
Figure 3: Hydroponic and salt tolerant studies in I. pes-caprae. Growth of shoots in HP solution (A); DW containing MS salts (B) and DW (C) after 10 days. Growth of shoots in HP solution (D); DW containing MS salts (E) and DW (F) after 27 days. Growth of shoots in 0.2 % NaCl supplemented HP solution (G); DW containing MS salts (H) and DW (I) after 27 days. Growth of shoots in 0.4 % NaCl supplemented HP solution (J); DW containing MS salts (K) and DW (L) after 27 days. Growth of shoots in 0.6 % NaCl supplemented HP solution (M); DW containing MS salts (N) and DW (O) after 27 days. Growth of shoots in 0.8 % NaCl supplemented HP solution (P); DW containing MS salts (Q) and DW (R) after 27 days.
*HP=hydroponic; DW=distilled water
Being a halophyte, I. pes-caprae plants were also tested for salt tolerance in above mentioned liquid solutions. Out of four conc. of NaCl used, 0.2 % and 0.4 % salt conc, favored the growth and the root and shoot development was found to be better than the solutions without supplementation of NaCl. The best growth in terms of strong leaves and thick roots was observed on 0.4 % NaCl supplemented-HP followed by 0.4 % NaCl supplemented-DW (Figure 3J-L). The higher salt conc. was found to be detrimental for shoot growth in all the solutions (Figure 3M-R).
Antifungal Activity of Methanolic Extract of I. pes-caprae
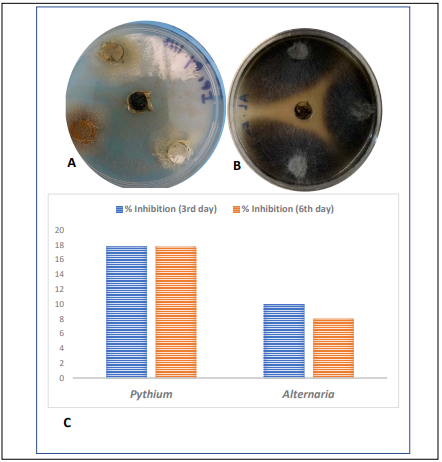
I. pes-caprae plant methanolic extract was found to show moderate activities against Pythium and Alternaria genus. The antifungal activity reveals that percent inhibition was maximum against Pythium sp. on 3rd day (17.80) followed by Alternaria sp. which showed maximum percent inhibition of 10.00 on third day. This activity of methanolic extract of Pythium was maintained also on 6th day also as Pythium fungus showed 17.71% inhibition compared to Alternaria which showed 8.034% inhibition. It is important to note here that the antifungal activities of the extract against Alternaria and Pythium reduced gradually from 3rd day to 6th day (Figure 4).
Figure 4: Ipomoea pes-caprae plant extract inhibition against Pythium (A) and Alternaria (B) respectively. Graph showing of percent inhibition of fungal strains Alternaria, and Pythium against plant extract of Ipomoea pes-caprae on 3rd and 6th day respectively (C).
Phytochemical Analysis of I. pes-caprae
UV Absorption Study
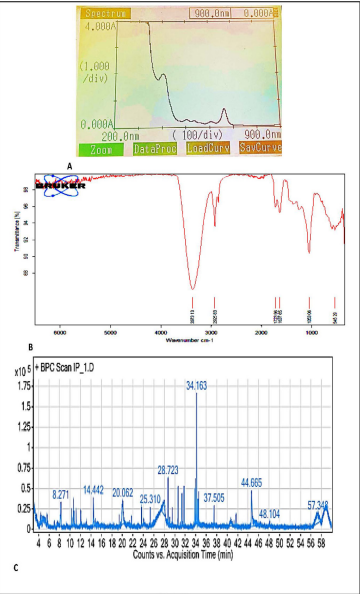
The UV-Vis phytochemical analysis of the methanolic extract in the range of 200-900nm revealed the presence of certain active functional groups based on the spectrum and peaks in the given range of study. Methanolic leaf extract (1%) showed absorbance between 0.054 to 1.967 between wave lengths 830-410 nm. One high absorbance peak (1.967) was visible in the UV (403.50nm) region, one mild absorbance peak (0.234) in the UV (534 nm) region, and one low absorbance peak (0.055) in the UV A (810-830nm) region (Figure 5A).
FTIR analysis
The FTIR spectrum obtained for the sample of I. pes- caprae (IP 1) revealed six major active functional groups, shown against the absorption peaks in the spectrum. The major active groups obtained are, OH Stretch (Wavenumber 3373.13cm-1), Alkane (Wavenumber 2925.83cm-1) as the group C-H stretching, and carbonyl at 1723.96cm-1 as C=O stretching. Alkene (C=C stretching) at Wavenumber 1637.65cm-1, Benzene (C-O Stretching) at Wavenumber 1053.06cm-1and halo compound obtained at Wavenumber 545.29cm 1 (Table 2; Figure 5B).
Table 2: Possible presence of functional group in sample of Ipomoea pes-caprae methanolic extract shown by FTIR analysis.
|
S.No. |
Major Peaks obtained |
Active Group |
Class of compound |
|
1 |
3373.13 |
O-H stretching |
OH Stretch |
|
2 |
2925.83 |
C-H stretching |
Alkane |
|
3 |
1723.96 |
C=O stretching |
Carbonyl |
|
4 |
1637.65 |
C=C stretching |
Alkene |
|
5 |
1053.06 |
C-O |
Benzene |
|
6 |
545.29 |
C-I stretching |
Halo compound |
GC-MS Analysis
The GC-MS chromatogram of I. pes-caprae showed the different peaks which are mentioned in the Table 3. There was no major difference between the presence of control and in vitro raised plants and a total of 26 major compounds could be identified based upon the clear peaks shown in the GC-MS chromatogram (Table 3; Figure 5C).
Table 3: Retention time and proposed compound based on GC-MS analysis of Ipomoea pes-caprae, methanolic leaf (in vitro) extract.
|
Peak |
RT |
Compound Name |
Area % |
|
1 |
5.615 |
Dihydoxyacetone |
1.38 |
|
2 |
8.264 |
Cyclohexan 1,1 dimethoxy |
3.01 |
|
3 |
10.304 |
4,5 diamini-2-hydroxypyrimdine |
2.17 |
|
4 |
10.638 |
Benzoic acid, methyl ester |
1.24 |
|
5 |
10.772 |
Undecane |
1.69 |
|
6 |
11.157 |
4H-pyran-4-one, 3-hydroxy-2-methyl |
0.96 |
|
7 |
12.03 |
2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one |
1.49 |
|
8 |
14.436 |
5-Hydroxymethylfurfural |
3.61 |
|
9 |
19.388 |
2-Amino-9-(3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-3,9-dihydro-puri |
0.53 |
|
10 |
20.056 |
unknown |
11.72 |
|
11 |
25.316 |
Cyclooctasiloxane, hexadecamethyl- |
1.00 |
|
12 |
27.485 |
Methoprene |
1.01 |
|
13 |
29.024 |
2-Pentadecanone, 6,10,14-trimethyl- |
0.82 |
|
14 |
30.551 |
7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione |
1.47 |
|
15 |
30.641 |
Hexadecanoic acid, methyl ester |
2.45 |
|
16 |
31.36 |
10-Methoxy-NB-Alpha-Methylcorynantheol |
3.38 |
|
17 |
31.764 |
unknown |
2.40 |
|
18 |
33.823 |
9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
5.11 |
|
19 |
33.932 |
9-Octadecenoic acid, methyl ester, (E)- |
8.93 |
|
20 |
34.08 |
8,11-Octadecadienoic acid, methyl ester |
0.36 |
|
21 |
34.163 |
Phytol |
9.12 |
|
22 |
34.407 |
Methyl stearate |
1.88 |
|
23 |
34.875 |
Oleic Acid |
0.51 |
|
24 |
35.51 |
Oleic Acid |
0.86 |
|
25 |
41.316 |
Phenol, 2,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl- |
1.36 |
|
26 |
44.665 |
Glycerol 1-palmitate |
31.56 |
Figure 5: Phytochemical analysis of in vitro raised glasshouse established Ipomoea pes-caprae plant. UV-Vis absorption spectrum (A); FTIR spectrum (B) and GC-MS chromatogram (C) of I. pes-caprae methanolic leaf extract.
DISCUSSION
In vitro Establishment of I. pes-carpae at Various Morphogenic Levels
The in vitro multiplication protocols have been considered a very important step in plant tissue culture studies as it provided a suitable media combination for the rapid growth of plants without any intervening phases. Though the in vitro establishment of I. pes-caprae has been conducted earlier studies and has revealed active role of 2-IP for shoot generation from nodal explants in I. pes-caprae [14]. The use of other hormone in the present study i.e., BAP in known for generation of shoot buds is also reported in many medicinal plants but interestingly higher concentration of this hormone may lead to callusing response also in certain cases [15].
Callus induction studies in this plant system has been conducted with an aim to introduce indirect regeneration and achievement of soma clonal variation so that new plant variety development in I. pes-caprae can be achieved. The use of several plant growth hormones has been conducted previously but the finding show that high concentrations of BAP have been found effective is inducing callusing response in the present studied whereas earlier conducted studies have recognized that 2-4 D and IAA (0.7mg/L) with the presence of 15% coconut water in half strength MS media was best suited for induction of callus and further regeneration of shoots [11]. So far very less work on tissue culture aspects of I. pes-caprae has been reported in the literature, therefore the present optimization of various media combinations for different morphogenic response will add up relevant information regarding I. pes-caprae in vitro establishment.
Hydroponic Establishment and Salt Tolerance Study of I. pes-caprae Plants
The hydroponic studies have been assessed in this plant system with a range of salt concentration as this plant was known to be a salt tolerant and sand binder agent and it was considered apt to check the higher as well as lower range of salt concentration’s effect on I. pes-caprae plant. The drying and yellowing of leaves in DWMS could have been due to presence of high salt concentration. The lethal and stress causing effect of MS salts on plant growth in deep water culture has also been mentioned in previous studies which showed optimum growth in hydroponic Hoagland solution [16]. The reason for hydroponic solution to perform better could be due to easy availability of ionic nutrients for the plant growth as compared to MS salts and distilled water. I. pes-caprae is a known salt tolerant plants and can grow in high water conditions such as near the beaches. Henceforth it may be considered that this plant system could be potentially salt tolerant variety which can be used as a sand binder for salinized areas. Previously also, study on various concentration of NaCl have been shown to have effect on the leaves, roots and stem segments in I. pes- caprae plants with parameter studies of analysis of protein content, presence of free amino acids, glycine betaine and proline content in the same plant system [17]. Up to a concentration of 200 Mm NaCl, showed an increment in the protein content but interestingly presence of free amino acid was found be reduced in these saline conditions. The study also indicated that an elevated salt concentration (up to 500 Mm NaCl) increased the proline and glycine betaine concentrations which possibly could play a positive role in adapting to the high stress of salinity [17].
Antifungal Activity of Methanolic Extract of I. pes-caprae
The antifungal activities shown by the methanolic extract of Ipomea against major fungal genus is a promising indication that this plant or its content can be used against a range of fungal strains in future. Earlier studies also have shown that other species of Ipomea such as Ipomea batatas has shown the confirmatory presence of certain compounds which have shown the antifungal activities against different fungal strains [18].
Phytochemical Analysis of I. pes-caprae
The invasive phytochemical investigations a prerequisite for this plant system to be used commercially as this in-depth analysis will help in gathering more information about the active phytomolecules present in the plants as well as their chemical characterization will help in using these molecules for various other activity-based analysis. Also, previous phytochemical analysis of plant methanolic extract have also shown the presence of tannins, phenolics and diterpenes which has provided an indication that this plant system can be used to develop a safe, herbal, affordable and effective sun screen [19]. Interestingly the methanolic extract of the leaves have shown comparable results with a known sun screen component Dermatone when Sun Protection Factor (SPF) was evaluated [19].
CONCLUSION
The optimization of in vitro propagation protocol and rapid hydroponic based multiplication has been optimized in I. pes-caprae for the first time in detail along with the phytochemical analysis. Extract based activity of the plant also showed antifungal activities that add up to its already mentioned medicinal value. The salt tolerant studies have provided important indication to use this plant in the highly saline conditions for soil amendment efforts.
ACKNOWLEDGMENTS
Authors highly acknowledge the help rendered by Dr. Hamed from Department of Agriculture and Marine Sciences (SQU, Oman) for providing fungal cultures.
AUTHOR CONTRIBUTIONS
SAK conceptualized the problem, conducted and interpreted the experiments. Sara and Mira assisted in in vitro investigations and analysis. MA assisted in UV absorption studies. FJ assisted in interpretation of various analysis; SSDP helped to perform and interpret the GC-MS analysis. Manuscript has been written and reviewed by SAK and PV.
FUNDING SOURCE
The work conducted has been supported by Internal Research Fund (IRF01) UTAS-Sur, Oman.
COMPETING INTERESTS
Authors declare no conflict of interests.
REFERENCES
- Miller G, Miranda M. (1988). Plants of Dhofar. Oman. 112.
- Ou-Yang PY, Liu N, Zhang WW, Wang J, Jian SG. (2011). Biological and eco-physiological characteristics of a beach plant Ipomoea pes caprae. J Hunan Univ Sci Technol Nat Sci Ed. 26:117–121.
- Kamenev M. (2011). Top 10 Aboriginal bush medicines. Australian Geographic.
- Akinniyi G, Jeonghee L, Hiyoung K, Joon-Goo L, Inho Y. (2022). A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A review of its botany, traditional uses, phytochemistry, and bioactivity. Marine Drugs. 20(5): 329.
- Maria De Souza M, Madeira A, Berti C, Krogh R, Yunes RA, et al. (2000). Antinociceptive properties of the methanolic extract obtained from Ipomoea pes-caprae (L.) R. Br. J Ethnopharmacol. 69:85–90.
- Bragadeeswaran S, Prabhu K, Sophia Rani S, Priyadharsini S, Vembu N. (2010). Biomedical application of beach morning glory Ipomoea pes-caprae. Int J Tropical Med. 5: 81-85.
- Kroth R, Berti C, Madeira AO, Souza MM, Cechinel-Filho V, Delle-Monache F, et al. (1999). Isolation and identification of compounds with antinociceptive action from Ipomoea pes-caprae (L.) R. Br. Die Pharmazie. 54:464-466.
- Meira M, da Silva EP, David JM, David JP. (2012). Review of the genus Review of the genus Ipomoea: chemistry and biological activities. Rev Bras Farmacogn. 22: 682–713.
- Panichakul T, Ponnikorn S, Tupchiangmai W, Haritakun W, Srisanga K. (2022). Skin anti-aging potential of Ipomoea pes-caprae ethanolic extracts on promoting cell proliferation and collagen production in human fibroblasts (CCD-986sk Cells). Pharmaceuticals. 15: 969.
- Li Y, Zhang Y, Fend D, Liang D, Cheng L, Ma F, et al. (2010). Overexpression of a Malus vacuolar Na/H antiporter gene (MDNHX1) in apple rootstock M.26 and its influence on salt tolerance. PCTOC. 102:337-345.
- Thirunavukkarasu P, Ramanathan T, Umamaheshwari G, Manigandan V, Dinesh P. (2014). Low-cost material enhanced the in vitro regeneration and micro propagation of medicinal sand dune plant species Ipomoea Pes-caprae (L.) R. Br. American J Plant Physiol. 9:16-23.
- Velazquez-Gonzalez RS, Garcia-Garcia AL, Ventura-Zapata E, Barceinas-Sanchez JDO, Sosa-Savedra JC. (2022). A review on hydroponics and the technologies associated for medium- and small-scale operations. Agriculture. 12:646.
- Murashige T, Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plant. 15:473-497.
- Kane ME, Bird KT, Lee TM. (1993). in vitro Propagation of Ipomoea Pes-Caprae (Railroad-Vine). J Coastal Res. 9: 356-362.
- Verma P, Khan SA, Mathur AK. (2022). De novo shoot bud induction from the Catharanthus roseus leaf explants and Agrobacterium tumefaciens-mediated technique to raise transgenic plants. In: Courdavault, V., Besseau, S. (eds) Catharanthus roseus. Methods in Molecular Biology. vol 2505. Humana, New York, NY.
- Van Delden SH, Nazarideljou MJ, Marcelis LFM. (2020). Nutrient solutions for Arabidopsis thaliana: A study on nutrient solution composition in hydroponics systems. Plant Methods. 16:72.
- Venkatesan A, Chellappan KP. (1998). Accumulation of proline and glycine betaine in Ipomoea pes-caprae induced by NaCl. Biologia Plantarum. 41: 271–276.
- Oluyori AP, Shaw AK, Preeti R, Reddy S, Atolani O, Olatunji GA, et al. (2016). Natural antifungal compounds from the peels of Ipomoea batatas Lam. Nat Prod Res. 18:2125-2129.
- Ratnasooriya WD, Pathirana RN, Dissanayake AS, Samanmali BLC, Banu RS. (2017). Methanolic leaf extract of Ipomoea Pes-caprae possesses in vitro sunn 13 screen activity. Imperial J Interdisciplinary Res. 3:2454-1362.
 Abstract
Abstract  PDF
PDF