Past Issues
Inhibitory Effect of Rhei Rhizoma Constituents on the Cortisol Production in ACTH-Stimulated Bovine Adrenal Fasciculata Cells
Naoko Kuwabara1,2, Yukiko Matsuo3, Tsuyoshi Ueki2, Yuki Nakamura3, Nanami Kase3, Taisuke Okayasu2, Yoshihiro Mimaki3, Eiichi Tachikawa4, Shinji Sato5, Haruki Yamada6, Saori Nakagawa1*
1 Department of Bio-analytical Chemistry, Faculty of Pharmacy, Niigata University of Pharmacy and Applied Life Sciences, Niigata, Japan
2 Department of Endocrine & Neural Pharmacology, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Tokyo, Japan
3 Department of Medicinal Pharmacognosy, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Tokyo, Japan
4 Department of Clinical Pharmaceutics and Pharmacy Practice, School of Pharmacy, Iwate Medical University, Japan
5 Department of Functional and Analytical Food Sciences, Faculty of Applied Life Sciences, Niigata University of Pharmacy and Applied Life Sciences, Niigata, Japan
6 School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Tokyo, Japan
*Corresponding author: Saori Nakagawa, Department of Bio-analytical Chemistry, Faculty of Pharmacy, Niigata University of Pharmacy and Applied Life Sciences, 265-1 Higashijima, Akiha-ku, Niigata 956-8603, Japan. Tel: +81-250-25-5296, E-mail: [email protected]
Naoko Kuwabara and Yukiko Matsuo contributed equally to this work.
Citation: Nakagawa S, et al. (2022). Inhibitory Effect of Rhei Rhizoma Constituents on the Cortisol Production in ACTH-Stimulated Bovine Adrenal Fasciculata Cells. Traditional Medicine. 3(2):10.
Received Date: Aug 31, 2022
Published Date: Sep 15, 2022
Copyrights: Nakagawa S, et al. © (2022).
ABSTRACT
Depression can be activated by dysfunction of the hypothalamus–pituitary–adrenal (HPA) axis and is sometimes accompanied by increased cortisol production. In this study, the effects of Kampo extract products on cortisol production in bovine adrenal fasciculata cells via adrenocorticotropic hormone (ACTH) stimulation were investigated with the aim of identifying substances that can improve the function of the HPA axis. Daiokanzoto extract product, which is prepared from Rhei Rhizoma and Glycyrrhizae Radix as the constituent crude drugs, was found to potently inhibit cortisol production in bovine adrenal fasciculata cells. Because only Rhei Rhizoma decoction showed potent inhibitory effect, the cortisol production inhibitory substances in Rhei Rhizoma was analyzed. As a result, six anthraquinones: chrysophanol, emodin, aloe-emodin, rhein, chrysophanol-8-O-β-D-glucopyranoside, and physicion-8-O-β-D-glucopyranoside were isolated and identified from Rhei Rhizoma decoction. Among these compounds, emodin showed the strongest inhibition of the ACTH-induced cortisol production in cells (IC50 = 13.1 ± 1.9 µM). These results suggest that emodin may help of stress-induced illnesses such as depression.
Keywords: cortisol; adrenal fasciculata cell; ACTH; Rhei Rhizoma; emodin
INTRODUCTION
People living in modern society experience various stresses in daily life. When the body is exposed to stress, the hypothalamus–pituitary–adrenal (HPA) axis is activated, and glucocorticoids such as cortisol are produced in large quantities to counteract the stress [1]. Moderate stress leads to the activation of vital responses, but excessive or chronic stress leads to excessive secretion of cortisol, which can cause mental, metabolic, and cardiovascular disorders [1, 2]. It has been reported that abnormal, excessive activation of the HPA axis is observed in approximately half of individuals with depression, and some patients exhibit increased cortisol production [3]. Therefore, it is considered that an inhibitory substance that appropriately regulates cortisol production might contribute to the prevention of stress-related illness and identification of such a substance might enable the development of a therapeutic drug.
In this study, to discover potential natural products that can improve the function of the HPA axis, the inhibitory activities of clinically applied Kampo extract products on adrenocorticotropic hormone (ACTH)-induced cortisol production in bovine adrenal fasciculata cells were randomly screened. As a result, Daiokanzoto extract product, which is prepared from consists of Rhei Rhizoma and Glycyrrhizae Radix as the constituent crude drugs, strongly inhibited the cortisol production in bovine adrenal fasciculata cells. In addition, Rhei Rhizoma decoction showed the strong inhibitory activity, whereas Glycyrrhizae Radix decoction showed no inhibitory activity.
Based on the above findings in this study, Rhei Rhizoma was phytochemically examined to identify the active ingredients contributing to the inhibition of cortisol production in ACTH-stimulated bovine adrenal fasciculata cells.
MATERIALS AND METHODS
General experimental procedures
NMR spectral data were measured by a DRX-500 spectrometer (Bruker, Karlsruhe, Germany) at 500 MHz for 1H NMR and 125 MHz for 13C NMR, using the standard pulse programs at 300 K. Chemical shifts were recorded as δ values with reference to tetramethylsilane as an internal standard. High resolution-electrospray ionization-time of flight-mass spectrometry data were obtained on an LCT mass spectrometer (Waters-Micromass, Manchester, UK). Column chromatography (CC) was performed using Diaion HP-20 (Mitsubishi Chemical, Tokyo, Japan), Sephadex LH-20 (GE Healthcare Life Sciences, Buckinghamshire, UK), silica gel Chromatorex BW-300 (Fuji Silysia Chemical, Aichi, Japan), and ODS silica gel COSMOSIL 75C18-OPN (Nacalai Tesque, Kyoto, Japan). TLC was performed using precoated silica gel 60F254 and RP18 F254S plates (0.25 mm thick; Merck, Darmstadt, Germany), and the spots were displayed by spraying the plates with 10% H2SO4 aqueous solution and then heating. HPLC was performed using a system comprising a pump (DP-8020; Tosoh, Tokyo, Japan), detector (RI-8020; Tosoh), and injection port (Rheodyne, Rohnert Park, CA, USA). A TSK gel ODS-100Z column (10 mm i.d. × 250 mm, 5 µm; Tosoh) was used for the preparative HPLC.
Crude drugs
Rhei Rhizoma (Lot. D9T0301) and Glycyrrhizae Radix (Lot. D0D3255) were obtained from Uchida Wakan-Yaku (Tokyo, Japan). The voucher specimens have been deposited at the Herbarium of the Tokyo University of Pharmacy and Life Sciences (Rhei Rhizoma: KS-2017-008; Glycyrrhizae Radix: KS-2018-014).
Preparation of test samples
Daiokanzoto extract product (TJ-84, Lot. K11442) was obtained from Tsumura (Tokyo, Japan) and was dissolved in dimethyl sulfoxide (DMSO). Rhei Rhizoma (5.0 g) and Glycyrrhizae Radix (5.0 g) were independently decocted with 600 mL of distilled water for 90 min, and extract was filtered through paper. The filtrate was lyophilized and dissolved in distilled water.
Extraction and isolation from Rhei Rhizoma
Rhei Rhizoma (dry weight, 5.0 kg) was extracted with H2O (8 L). After the solvent was removed using an evaporator, the concentrated H2O extract (575 g) was fractionated using a Diaion HP-20 column (2400 g, 85 mm i.d. × 600 mm) with MeOH/H2O (3:7; 1:1), MeOH, EtOH, and EtOAc as the eluent. The EtOH-eluted portion (3.2 g) was subjected to silica gel CC (2000 g, 32 mm i.d. × 590 mm) eluted with hexane/EtOAc (10:1; 1:1; 1:10) and EtOAc/MeOH/H2O (20:3:2) to obtain 4 sub-fractions (Frs. A–D). Fraction A was separated by ODS silica gel CC (180 g, 15 mm i.d. × 390 mm) using MeCN/H2O (3:1) to obtain 1 (31.0 mg). Fr. B was chromatographed on silica gel (420 g, 29 mm i.d. × 450 mm) eluted with CHCl3/MeOH/H2O (40:2:1; 200:10:1) and ODS silica gel (200 g, 17 mm i.d. × 600 mm) eluted with MeCN/H2O (2:1; 3:1; 4:1) to yield 2 (45.4 mg). Fr. C was separated by ODS silica gel CC (180 g, 15 mm i.d. × 390 mm) using MeCN/H2O (1:1) and MeOH/H2O (3:2; 3:1) and preparative TLC using CHCl3/MeOH/H2O (100:10:1) to yield 3 (7.8 mg). Fr. D was subjected to silica gel CC (420 g, 29 mm i.d. × 450 mm) eluted with CHCl3/MeOH/H2O (100:10:1; 100:50:1), ODS silica gel CC (200 g, 17 mm i.d. × 600 mm) eluted with MeCN/H2O (1:3; 1:1; 2:1), Sephadex LH-20 CC (75 g, 13 mm i.d. × 470 mm) eluted with MeOH/H2O (2:1; 3:1), and preparative HPLC using MeOH/H2O (1 : 1) to obtain 4 (5.8 mg), 5 (33.4 mg), and 6 (5.8 mg).
Structural characterization
Chrysophanol (1): 1H NMR (500 MHz, C5D5N) δH: 7.19 (br s, H-2), 7.76 (br s, H-4), 7.95 (dd, J = 7.5, 1.0 Hz, H-5), 7.67 (dd, J = 8.4, 7.5 Hz, H-6), 7.40 (dd, J = 8.4, 1.0 Hz, H-7), 2.29 (br s, Me); 13C NMR (125 MHz, C5D5N) δC: 162.8 (C-1), 124.5 (C-2), 150.2 (C-3), 121.3 (C-4), 134.1 (C-4a), 119.8 (C-5), 137.3 (C-6), 124.6 (C-7), 162.6 (C-8), 116.4 (C-8a), 192.6 (C-9), 114.2 (C-9a), 181.9 (C-10), 133.7 (C-10a), 21.8 (Me); HR-ESI-TOF-MS m/z: 255.0657 [M+H]+ (calcd for C15H11O4, 255.0657).
Emodin (2): 1H NMR (500 MHz, CD3OD) δH: 7.08 (dd, J = 1.6, 0.9 Hz, H-2), 7.56 (dd, J = 1.6, 0.5 Hz, H-4), 7.17 (d, J = 2.5 Hz, H-5), 6.55 (d, J = 2.5 Hz, H-7), 2.42 (br s, Me); 13C NMR (125 MHz, CD3OD) δC: 162.8 (C-1), 124.5 (C-2), 150.2 (C-3), 121.3 (C-4), 134.1 (C-4a), 119.8 (C-5), 137.3 (C-6), 124.6 (C-7), 162.6 (C-8), 116.4 (C-8a), 192.6 (C-9), 114.2 (C-9a), 181.9 (C-10), 133.7 (C-10a), 21.8 (Me); HR-ESI-TOF-MS m/z: 271.0606 [M+H]+ (calcd for C15H11O5, 271.0606).
Aloe-emodin (3): 1H NMR (500 MHz, C5D5N) δH: 7.60 (br s, H-2), 8.16 (br s, H-4), 7.35 (br d, J = 7.4 Hz, H-5), 7.61 (dd, J = 8.4, 7.4 Hz, H-6), 7.90 (br d, J = 8.4 Hz, H-7), 5.01 (br s, CH2), 11.93 (s, 8-OH); 13C NMR (125 MHz, C5D5N); δC: 162.7 (C-1), 121.6 (C-2), 163.1 (C-3), 117.7 (C-4), 134.1 (C-4a), 119.5 (C-5), 137.1 (C-6), 124.0 (C-7), 162.9 (C-8), 116.6 (C-8a), 192.3 (C-9), 115.2 (C-9a), 182.1 (C-10), 134.3 (C-10a), 63.3 (CH2); HR-ESI-TOF-MS m/z: 293.0426 [M+Na]+ (calcd for C15H10NaO5, 293.0426).
Rhein (4): 1H NMR (500 MHz, DMSO) δH: 7.80 (d, J = 1.5 Hz, H-2), 8.30 (d, J = 1.5 Hz, H-4), 8.34 (dd, J = 7.6, 1.2 Hz, H-5), 7.76 (dd, J = 8.3, 7.6 Hz, H-6), 7.82 (dd, J = 8.3, 1.2 Hz, H-7); HR-ESI-TOF-MS m/z: 307.0219 [M+Na]+ (calcd for C15H8NaO6, 307.0219).
Chrysophanol-8-O-β-D-glucopyranoside (5): 1H NMR (500 MHz, C5D5N) δH: 7.14 (dd, J = 1.6, 0.8 Hz, H-2), 7.69 (dd, J = 1.6, 0.5 Hz, H-4), 8.07 (dd, J = 7.6, 1.0 Hz, H-5), 7.62 (dd, J = 8.4, 7.6 Hz, H-6), 8.01 (dd, J = 8.4, 1.0 Hz, H-7), 2.23 (br s, Me), Glc-1’-6’: 5.81 (d, J = 7.7 Hz) 4.57 (dd, J = 9.1, 7.7 Hz), 4.44 (dd, J = 9.1, 9.0 Hz), 4.38 (dd, J = 9.2, 9.0 Hz), 4.25 (ddd, J = 9.2, 5.6, 2.3 Hz), 4.62 (dd, J = 11.4, 5.6 Hz), 4.43 (dd, J = 11.4, 2.3 Hz); 13C NMR (125 MHz, C5D5N) δC: 162.9 (C-1), 124.6 (C-2), 147.8 (C-3), 121.2 (C-4), 132.9 (C-4a), 121.7 (C-5), 136.0 (C-6), 123.1 (C-7), 159.4 (C-8), 120.0 (C-8a), 188.6 (C-9), 115.5 (C-9a), 182.6 (C-10), 135.1 (C-10a), 21.7 (Me), Glc-1’-6’:102.8, 74.9, 78.4, 71.1, 79.4, 62.5; HR-ESI-TOF-MS m/z: 439.1002 [M+Na]+ (calcd for C21H20NaO9, 439.1005).
Physicion-8-O-β-D-glucopyranoside (6): 1H NMR (500 MHz, C5D5N) δH: 7.15 (dd, J = 1.6, 0.8 Hz, H-2), 7.69 (dd, J = 1.6, 0.4 Hz, H-4), 7.65 (d, J = 2.6 Hz, H-5), 7.61 (d, J = 2.6 Hz, H-7), 2.23 (br s, Me), 3.83 (s, OMe), Glc-1’-6’: 5.78 (d, J = 7.7 Hz), 4.57 (dd, J = 8.6, 7.7 Hz), 4.42 (dd, J = 8.7, 8.6 Hz), 4.31 (dd, J = 9.4, 8.7 Hz), 4.24 (ddd, J = 9.4, 6.3, 2.6 Hz), 4.37 (dd, J = 11.2, 6.3 Hz), 4.65 (dd, J = 11.2, 2.6 Hz); 13C NMR (125 MHz, C5D5N) δC: 162.0 (C-1), 124.7 (C-2), 147.3 (C-3), 120.1 (C-4), 133.0 (C-4a), 107.3 (C-5), 165.6 (C-6), 108.3 (C-7), 161.9 (C-8), 115.7 (C-8a), 187.6 (C-9), 115.4 (C-9a), 182.6 (C-10), 137.3 (C-10a), 21.6 (Me), 56.0 (OMe) Glc-1’-6’:103.1, 74.9, 79.6, 71.4, 78.5, 62.6; HR-ESI-TOF-MS m/z: 469.1114 [M+Na]+ (calcd for C22H22NaO10, 469.1111).
Isolation and primary culture of bovine adrenal fasciculata cells
Bovine adrenal glands were kindly provided by Kanagawa Meat Center (Japan). Adrenal fasciculata cells were prepared by collagenase digestion as previously described [4]. The isolated cells were suspended in a 1:1 mixture of Dulbecco’s modified Eagle medium (DMEM) and Ham’s F-12 (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), and antibiotics [penicillin (50 µg/mL), streptomycin (50 µg/mL), neomycin (100 µg/mL), and amphotericin B (250 µg/mL) (Thermo Fisher Scientific)] were maintained as a monolayer culture in plates with 15-mm diameter wells at a density of 5×10 5 cells. The cells were cultured in a CO2 incubator (95% air, 5% CO2) at 37°C.
Measurement of cortisol in bovine adrenal fasciculata cells
Oxygenated Krebs–Ringer–HEPES (N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid]) (KRH) buffer (pH 7.4) was composed of 125 mM NaCl, 4.8 mM KCl, 2.6 mM CaCl2, 1.2 mM MgSO4, 5.6 mM glucose (Fujifilm Wako Pure Chemical, Osaka, Japan), 25 mM HEPES (Dojindo Laboratories, Kumamoto, Japan), and 0.1% bovine serum albumin (Sigma-Aldrich) [4, 5]. This buffer was used as the reaction medium. After 48 h of culture, the cells were washed twice with KRH buffer and incubated with or without test compounds in the presence or absence of human ACTH [1–24] (Seikagaku Kogyo, Tokyo, Japan) (1 nM) in 1 mL of KRH buffer at 37°C for 90 min. Five fractions (MeOH/H2O [3:7; 1:1], MeOH, EtOH, and EtOAc) and compounds (1, 2, and 4-6) were dissolved in DMSO. DMSO in the reaction medium was at a final concentration of 0.5%, which showed no effect on the productions of cortisol and no cytotoxicity against to bovine adrenal fasciculata cells under the conditions used in this study. Trilostane (Toronto Research Chemicals) (10 μM), an inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD), was used as a positive control [6,7]. The reaction was terminated by transferring the reaction medium to tubes. The cortisol produced in the medium was extracted with dichloromethane (Fujifilm Wako Pure Chemical) and quantified by the sulfonic acid condensation method, using a fluorescence spectrophotometer (650-10S; Hitachi High-Technologies, Tokyo, Japan) at an excitation wavelength of 470 nm and an emission wavelength of 520 nm [8]. The amount of cortisol produced from the cells was expressed as ng/90 min/well. The values of the basal cortisol production were subtracted from the data, and ACTH-induced cortisol production was assigned a value of 100% as the control. The IC50 value, i.e., the concentration of a compound necessary to reduce the cortisol production by 50% compared to the control, was determined by dose response curves. The test compounds were evaluated for cytotoxicity using Cell Counting Kit-8 (Dojindo Laboratories), and the experiment was performed using a concentration of bovine adrenal fasciculata cell viability of 80% or more.
Statistical analyses
Data are expressed as the means ± standard error of mean values. Statistical analysis was performed using the statistical software package EZR, version 1.40 [9]. A value of p < 0.05 was considered statistically significant by Tukey-Kramer test.
RESULTS AND DISCUSSION
We investigated effects of Kampo extract products on cortisol production in bovine adrenal fasciculata cells stimulated with ACTH at concentrations of up to 200 µg/mL. Among the Kampo extract products tested, Daiokanzoto, which consists of Rhei Rhizoma and Glycyrrhizae Radix, strongly inhibited the cortisol production with an IC50 of 78.8 ± 8.0 µg/mL. Rhei Rhizoma decoction strongly inhibited the ACTH-induced production of cortisol in a dose-dependent manner (data not shown), and its IC50 value was calculated as 19.2 ± 2.8 µg/mL, whereas Glycyrrhizae Radix decoction showed no inhibitory effect at 200 µg/mL. These findings prompted us to investigate the chemical constituents of Rhei Rhizoma to determine the main substance(s) responsible for the cortisol production inhibitory effect on bovine adrenal fasciculata cells stimulated with ACTH. It has been reported that trilostane is a competitive inhibitor of the 3β-HSD, which is essential for synthesis of cortisol and all other steroids [6, 7]. Therefore, trilostane was used as a positive control for the inhibitory substance. Trilostane showed strongly inhibitory effect on cortisol produced by ACTH (p <0.01; Figure 1).
Figure 1: Effects of MeOH/H2O (3:7; 1:1), MeOH, EtOH, and EtOAc eluted fractions from Rhei Rhizoma on ACTH-induced cortisol production in bovine adrenal fasciculata cells.
Cultured cells were incubated with different concentrations of five fractions prepared from Rhei Rhizoma decoction or trilostane (10 µM) in the presence or absence of 1 nM ACTH at 37°C for 90 min. The ACTH-induced cortisol level was assigned a value of 100% as the control. Values represent means ± standard error of mean (S.E.M.) (n=3). **p<0.01, *p<0.05 vs. the control by Tukey-Kramer test.
First, we have examined the effects of the five fractions prepared from Rhei Rhizoma extract using a Diaion HP-20 column on the cortisol production in bovine adrenal fasciculata cells. When the cells were incubated MeOH/H2O (3:7 or 1:1) eluted fractions, respectively, ACTH-stimulated cortisol production was slightly decreased. In contrast, the cells treated with MeOH, EtOH, or EtOAc eluted fractions, respectively, showed significant decreases in cortisol production (p <0.01; Figure 1). The EtOH eluted fraction showed a particularly strong effect and was thus further separated by a series of chromatographic methods, thereby obtaining compounds 1-6 (Figure 2).
Figure 2: Structures of compounds 1-6 isolated from the EtOH fraction of Rhei Rhizoma decoction. Chrysophanol (1), emodin (2), aloe-emodin (3), rhein (4), chysophanol-8-O-β-D-glucopyranoside (5), and physicion-8-O-β-D-glucopyranoside (6).
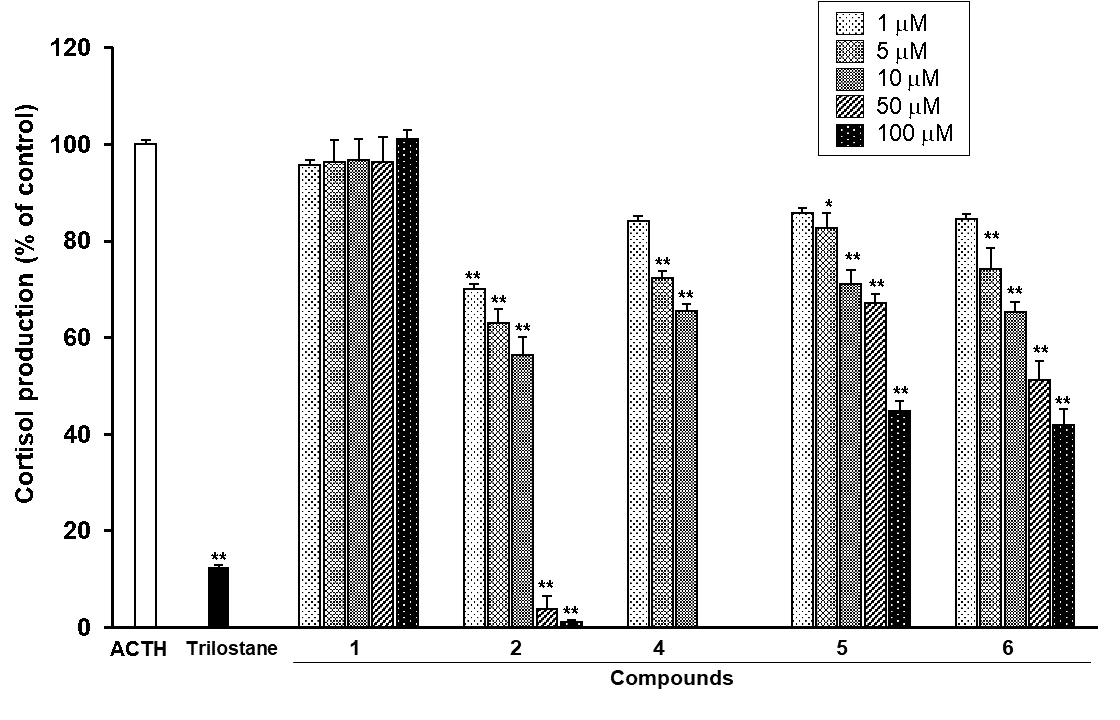
Compound 1-6 were identified as chrysophanol (1) [10-12], emodin (2) [11-13], aloe-emodin (3) [11, 12], rhein (4) [11, 12], chrysophanol-8-O-β-D-glucopyranoside (5) [14], and physicion-8-O-β-D-glucopyranoside (6) [14]) by comparing their physicochemical and spectroscopic data with literature values. Excluding aloe-emodin (3) because of its low recovery rate, the other five compounds (1, 2, and 4-6) were evaluated in terms of cortisol production inhibitory effect in bovine adrenal fasciculata cells stimulated with ACTH. Chrysophanol (1) had no effect on cortisol production. In contrast, emodin (2) inhibited cortisol production in a dose-dependent manner (0.1–50 µM), and its IC50 value was calculated as 13.1 ± 1.9 µM. The chemical structures of chrysophanol and emodin differed only in the presence or absence of a hydroxy group at the C-6 position. In a previous study, the polyphenols EGC, EGCG, and GA were reported to inhibit HMG lyase activity, and this was related to the presence or absence of a hydroxy group in the gallyl moiety [15]. Therefore, it is considered that the presence or absence of the hydroxy group is related to the inhibitory effect of the enzyme contributed to the cortisol production in bovine adrenal fasciculata cells. The IC50 of rhein (4) was not calculated because of its cytotoxicity. It has been reported that rhein induced apoptotic cell death in primary cultures of rat hepatocytes [16], and it was revealed to also show cytotoxicity against bovine adrenal fasciculata cells. Chysophanol-8-O-β-D-glucopyranoside (5) and physicion-8-O-β-D-glucopyranoside (6) also inhibited cortisol production with IC50 values of 40.0 ± 4.2 µM and 54.0 ± 3.2 µM, respectively, but their inhibitory effect was weaker than that of emodin (2) (Figure 3).
Figure 3: Effects of compounds 1, 2, and 4-6 isolated from the EtOH fraction of Rhei Rhizoma on the ACTH-induced cortisol production in bovine adrenal fasciculata cells.
Cultured cells were incubated with different concentrations of compounds 1, 2, and 4-6 isolated from the EtOH fraction or trilostane (10 µM) in the presence or absence of 1 nM ACTH at 37°C for 90 min. The ACTH-induced cortisol level was assigned a value of 100% as the control. Values represent means ± S.E.M. (n=3). **p<0.01, *p<0.05 vs. the control by Tukey-Kramer test.
Since chrysophanol (1) showed no inhibitory effect, chysophanol-8-O-β-D-glucopyranoside (5) may act in an extracellular manner.
CONCLUSION
The results of this study suggest that the cortisol production inhibitory effect on bovine adrenal fasciculata cells stimulated with ACTH is caused mainly by emodin (2), an anthraquinone derivative contained in Rhei Rhizoma, and emodin may be useful for the prevention of stress-induced disorders. Rhei Rhizoma is prepared from the rhizomes of some Rheum species (Polygonaceae) such as R. palmatum L., and is reported to show not only laxative and anti-inflammatory activity [17, 18], but also psychotropic activity [18]. Present results also may be important to find alternative use of Rhei Rhizoma. These results suggest that emodin may help of stress-induced illnesses such as depression.
ACKOWLEDGEMENTS
We would like to thank Hiromichi Sato for providing experimental assistance.
CONFLICTS OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
REFERENCES
- Möstl E, Palme R. (2002). Hormones as indicators of stress. Domest Anim Endocrinol. 23(1-2):67–74.
- Haucke M, Golde S, Saft S, Hellweg R, Liu S, et al. (2022). The effects of momentary loneliness and COVID-19 stressors on hypothalamic-pituitary adrenal (HPA) axis functioning: A lockdown stage changes the association between loneliness and salivary cortisol. Psychoneuroendocrinology. 145:10584.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, et al. (2002). Neurobiology of depression. Neuron. 34(1):13–25.
- Tachikawa E, Itho K, Kudo K, Harada K, Kashimoto T, et al. (1999). Effects of interferons on cortisol production in bovine adrenal fasciculata cells stimulated by adrenocorticotropin. J Pharm Pharmacol. 51(4):465–473.
- Hasegawa E, Nakagawa S, Miyate Y, Takahashi K, Ohta S, et al. (2013) Inhibitory effect of protopanaxatriol ginseng metabolite M4 on the production of corticosteroids in ACTH-stimulated bovine adrenal fasciculata cells. Life Sci. 92(12):687–693.
- José PFS, Bermejo CA, Alonso-Miguel D, Sanz SG, Moral IC, et al. (2022). Survival of dogs with pituitary-dependent hyperadrenocorticism treated twice daily with low doses of trilostane. Vet Rec. 191(3):e1630.
- Yamato S, Nakagawa S, Yamazaki N, Aketo T, Tachikawa E. (2010). Simultaneous determination of pregnenolone and 17α-hydroxypregnenolone by semi-micro high-performance liquid chromatography with an immobilized cholesterol oxidase as a pre-column reactor: application to bovine adrenal fasciculata cells. J Chromatogr B Analyt Technol Biomed Life Sci. 878(32):3358–3362.
- Silber RH, Busch RD, Oslapas R. (1958). Practical procedure for estimation of corticosterone or hydrocortisone. Clin Chem. 4(4):278–285.
- Kanda Y. (2013). Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 48(3):452–458.
- Prateeksha, Yusuf MA, Singh BN, Sudheer S, Kharwar RN, et al. (2019). Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 9(2):68.
- Danielsen K, Aksnes DW, Francis GW. (1992). NMR study of some anthraquinones from rhubarb. Magn Reson Chem. 30:359–360.
- Zhou X, Song B, Jin L, Hu D, Diao C, et al. (2006). Isolation and inhibitory activity against ERK phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg Med Chem Lett. 16(3):563–568.
- Francis GW, Aksnes DW, Holt O. (1998). Assignment of the 1H and 13C NMR spectra of anthraquinone glycosides from Rhamnus frangula. Magn Reson Chem 36:769–772.
- Zhang W, Ye M, Zhan J, Chen Y, Guo D. (2004). Microbial glycosylation of four free anthraquinones by Absidia coerulea. Biotechnol Lett. 26(2):127–131.
- Nakagawa S, Kojima Y, Sekino K, Yamato S. (2013). Effect of polyphenols on 3-hydroxy-3-methylglutaryl-coenzyme A lyase activity in human hepatoma HepG2 cell extracts. Biol Pharm Bull. 36(12):1902–1906.
- Kågedal K, Bironaite D, Ollinger K. (1999). Anthraquinone cytotoxicity and apoptosis in primary cultures of rat hepatocytes. Free Radic Res. 31(5):419–428.
- Sim Y, Oh H, Oh D-S, Kim N, Gu PS, et al. (2015). An experimental study on providing a scientific evidence for seven-time alcohol-steaming of Rhei Rhizoma when clinically used. BMC Complement Altern Med. 15:388.
- Nishioka I. (1996). Psychotropic effects of rhubarb. Jpn J Orient Med. 46(5):631–644.
 Abstract
Abstract  PDF
PDF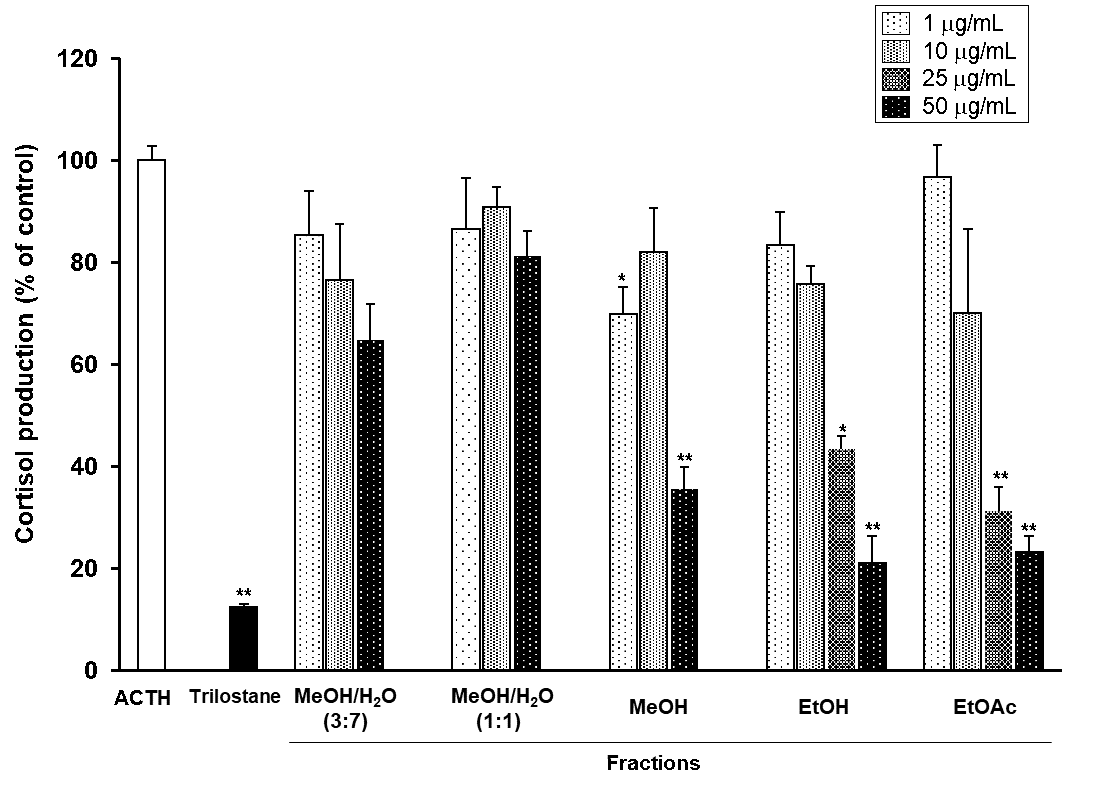
.png)