Past Issues
An Overview of Agarwood, Phytochemical Constituents, Pharmacological Activities, and Analyses
Shui-Tein Chen*, Yerra Koteswara Rao*
ALPS Biotech Co., Ltd., National Biotechnology Research Park, Nangang, Taipei City 11571, Taiwan.
*Corresponding authors:
Shui-Tein Chen ALPS Biotech Co., Ltd., National Biotechnology Research Park, Nangang, Taipei City 11571, Taiwan. Tel: +886-2-27887626; Email: [email protected]
Yerra Koteswara Rao ALPS Biotech Co., Ltd., National Biotechnology Research Park, Nangang, Taipei City 11571, Taiwan. Email: [email protected]
Citation: Chen ST, Rao YK. (2022). An Overview of Agarwood, Phytochemical Constituents, Pharmacological Activities, and Analyses. Traditional Medicine. 3(1):8.
Received: April 21, 2022
Published: July 17, 2022
ABSTRACT
Agarwood is a resin-impregnated heartwood obtained from the plants belongs to the genera, Aquilaria, Daphne, Gonystylus, Gyrinops and Wikstroemia. It is traditionally used for the production of perfume and incense stick, and pharmaceutical applications. Agarwood usually induced by the natural (traditional), conventional, and non-conventional methods. The major groups of phytochemicals identified in agarwood extracts are sesquiterpenes, 2-(2-phenylethyl)-4H-chromen-4-one derivatives (PECs), and aromatic compounds. These phytochemicals are showed various pharmalogical properties such as anti-inflammatory, cytotoxic, neuroprotective, anti-diabetic, anti-bacterial, etc. Several analytical techniques are applied to analyze the agarwood phytochemicals including sesquiterpenes, which exists mostly in the form of essential oils, and the fragrance constituents of PECs. The present review summarize the agarwood traditional uses, induction methods, phytochemical constituents, potential pharmacological activities, along with analyses methods. This review was carried out by searching various scientific databases, including Google Scholar, PubMed, Elsevier, ACS publications, Taylor and Francis, Wiley Online Library, MDPI, Springer, Thieme, and ProQuest. The present review provides a scientific basis for future studies and necessary information for the development of agarwood based therapeutic agents.
Keywords: Agarwood; Traditional uses; Induction methods; Chemical constituents; Biological activities; Analyses
INTRODUCTION
Agarwood, known as aloeswood or eaglewood, is an aromatic dark resin-impregnated heartwood obtained from wounded tree species of the Thymelaeaceae family [1]. The plant family Thymelaeaceae contains 54 genera, including Aquilaria, Daphne, Gonystylus, Gyrinops and Wikstroemia [1]. The species of the genus Aquilaria, Gonystylus, and Gyrinops produce agarwood [1]. In particular, the genus Aquilaria contains 57 species, among these 21 are accepted in the plant list [1]. So far, fifteen species of Aquilaria and nine species of Gyrinops are reported as agarwood producing plants [2]. Agarwood (resin)-producing species are found from the forests of Southeast Asia including, Bangladesh, Bhutan, China, India, Indonesia, Laos, Malaysia, Myanmar, Singapore, Taiwan, Thailand, and Vietnam [1,2]. They are usually found in lowland tropical forests with optimal sunlight, shade and moisture. Agarwood-producing species have a small flower similar to that of ‘jasmine’, and the fruit is bitter [3].
Healthy Aquilaria tree does not produce agarwood [2]. The healthy wood is white, soft, even-grained and not having a perfumed smell, as compared with the dark, hard and heavy scented characterictics resin-impregnated agarwood [2]. The agarwood resin developed through pathological, wounding and non-pathological mechanisms [4]. The formation of agarwood occurs naturally in response to natural injuries such as lightning, insects and mold attacks [4]. The deposited resin around the wounds over the years accumulate and eventually forms agarwood [4]. Therefore, Agarwood is termed as the resin-impregnated pieces of wood [4], and its formation is related to the self-defense mechanism of Aquilaria trees in response to biotic and abiotic stresses [1,2]. Stresses trigger the defense responses of Aquilaria species, which in turn initiate the secondary metabolite biosynthesis and the accumulation of agarwood resin [1,2].
The prominent species of agarwood producing Aquilaria species are, A. beccariana Tiegh., A. crassna Pierre ex Lecomte, A. filaria (Oken) Merr., A. hirta Ridl., A. khasiana Hallier f., A. malaccensis Lamk., A. microcarpa Baill., A. rostrata Ridl., A. sinensis (Lour.) Spreng., and A. agallocha [5]. Among these A. agallocha, A. crassna, A. malaccensis, and A. sinensis gain significant attention due to their therapeutic uses in traditional Southeast Asian medical systems [5]. Accordingly, these species appear frequently in the literature, particularly A. crassna, A. malaccensis and A. sinensis [1,5]. The agarwood producing Aquilaria species and their native place are presented in Table 1. With the increasing demand for agarwood, the population of agarwood species is declining rapidly in the wild. Currently, the genus Aquilaria is listed as endangered species and protected under Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) regulation [1]. The index of CITES species listed A. agallocha Roxb. as a synonym of A. malaccensis Lamk [1]. A. malaccensis Lamk., is also synonym to Aquilariella malaccensis (Lam.) Tiegh., and Agallochum malaccense (Lam.) Kuntze [6]. The International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species is listed A. crassna as critically endangered, and A. malaccensis and A. sinensis as vulnerable [7].
Table 1: Agarwood producing Aquilaria species and their place of origin.
|
Species |
Place of origin (Native) |
|
Aquilaria apiculata Merr |
Philippines |
|
Aquilaria baillonii Pierre ex Lecomte |
Cambodia, Thailand, Laos, Vietnam |
|
Aquilaria banaensis P.H.H6 |
Vietnam |
|
Aquilaria beccariana Tiegh |
Indonesia |
|
Aquilaria citrinicarpa (Elmer) Hallier f. |
Philippines |
|
Aquilaria crassna Pierre ex Lecomte |
Thailand, Cambodia, Vietnam |
|
Aquilaria cumingiana (Decne) Ridl |
Malaysia |
|
Aquilaria khasiana Hallier f. |
India |
|
Aquilaria malaccensis Lam. |
India, Myanmar, Malaysia, Indonesia, Philippines |
|
Aquilaria microcarpa Baill |
Indonesia |
|
Aquilaria parvifolia (Quisumb) Ding Hon |
Philippines |
|
Aquilaria rostrata Ridl |
Malaysia |
|
Aquilaria rugosa Kiet Kessler |
Vietnam |
|
Aquilaria sinensis (Lour.) Gilg |
China |
|
Aquilaria subintegra Ding Hon |
Thailand |
|
Aquilaria urdanetensis (Elmer) Hallier f. |
Philippines |
|
Aquilarla yunnanensis S.C. Huang |
China |
1.1. Traditional uses
Agarwood is known as “wood of God” because of religious practices [4]. The word “aloes” which means agarwood is found in the Sanskrit poet, Kâlidâsa, dated back to c. 4th–5th century CE [4]. Agarwood is considered the finest natural incense and has been used in many cultures, such as the Arabian, Chinese, Indian, and Japanese cultures [8]. Agarwood also associated with religious history, rituals and ceremonies in Buddhism, Christianity, Hinduism, and Islam [8]. It is known as gaharu in the Indonesia and Malaysia, jin-koh in Japan, chen xiang (沉香) in Chinese, agar in India, chim-hyuang in Korea, kritsana noi in Thailand, tram huong in Vietnam, and oud in the Middle East [8]. Agarwood is widely used as therapeutic perfumes, traditional medicine, religious purposes and aromatic food ingredient (Table 3) [3]. In the traditional Chinese and Ayurvedic medicines as an aphrodisiac, sedative, cardiotonic and carminative, as well as to treat gastric problems, coughs, rheumatism and high fever [9]. Agarwood is a traditional Chinese medicine included in the 2020 edition of Chinese Pharmacopoeia [10]. In traditional Arabian medicine, agarwood essential oil is used for aromatherapy [3]. In Thailand, agarwood has been used for a long time as a traditional treatment for infectious diseases such as diarrhea and skin diseases [3]. Additionally, A. crassna extract has been using as the ingredient of Ya-hom, a traditional Thai herbal formulation for the treatment of fainting by targeting the cardiovascular system.
1.2. Grading system
The market price of agarwood has commercial attention. However, the grading process of agarwood is largely depends on the human experience from the age-old practices of each country [1]. In general, the classification of agarwood oil quality is based on wood physical properties, long lasting aroma when burnt, color, resin content, high fixative properties and consumer perception, etc [1]. The higher the grade of agarwood, the richer the layers of aroma [1]. The best agarwood fragrance is mellow and sweet, full of penetration and persistence, and the powdery waxy material on the surface can be scraped off and kneaded it into a ball [1]. Its aroma is regarded as a symbol of high quality. Complexity and variability in agarwood composition are major challenges associated with its grading process. The morphological grading system of agarwood is shown in Table 2.
Table 2: Assesment of agarwood quality using grading system.
|
Observational Feature |
Grading Categary |
||
|
A |
B |
C |
|
|
Sense of oiliness |
Strong |
Strong |
Mild |
|
Aroma |
Strong, feel sweet and cool |
Less potent odor, feel sweet and slightly spicy |
Mild aroma, feel slightly sweet, salty |
|
Resin density |
High dense, compact, sink in water when soaked |
Dense, less compact, half-sinkage in water |
Light and not dense, full-floating on water |
|
Weight |
Hard texture, brittle, and not hollowed |
Texture little hard, little brittle, slightly hollow |
Loose texture, not brittle, and hollow |
1.3. Economical value
Agarwood is a valuable, non-timber forest product used different societies for medicinal, aromatic, cultural and religious purposes [8]. As the wealth of the consumer countries are gradually increased in recent decades, the market’s demand for agarwood started to exceed its supply [1]. The market value of agarwood derivative products is dependent on the classification or grading of agarwood, which is determined by a cumulative factor of the fragrance strength and longevity, resin content, geographical origin and purity (for oil) [8]. Global agarwood prices can be ranging from US$20 – 6,000 per/kg for the wood chips depending on its quality or US$ 10,000 per/kg for the wood itself [1]. High quality wood is used as incense in Arabian households and for the ‘koh-doh’ incense ceremony in Japan [8]. High-quality agarwood products can reach prices as high as US$100,000/kg. In the form of oud oil which is distilled from agarwood for perfumery, can be sold for US $1500 per 11.7 g [1,9]. The annual global market for agarwood has been estimated to be in the range of US$ 6 – 8 billion [1,9]. Agarwood has commercial importance in three categories i.e. perfume production, incense stick, and pharmaceuticals as described below [8].
1.3.1. Perfume
Agarwood oil is an essential oil obtained by water and steam distillation of agarwood [1]. Agarwood oil is a yellow to dark amber, viscous liquid with a characteristic balsamic and woody odour [9,11]. It is used in luxury perfumery for application. Agarwood perfumes are commonly prepared in both alcoholic and non-alcoholic carriers [9,11]. Agarwood perfume has a unique smell obtained from fragrance essential oil and aromatic compound [11]. The oil is also used as a fragrance in the production of cosmetics and personal care products, such as soaps and shampoos [9,11]. Agarwood resin is a key ingredient in old and new Arabic perfume products, and used as an element within high-quality perfumes in Arabic, Japanese and Indian cultures [4]. Traditionally in the Middle East, agarwood oil is used as a scent, and Minyak attar (water-based) [4]. “Attar” is an example of a water-based perfume containing agarwood oil, which is traditionally used by Muslims to lace prayer clothes [4]. Agarwood oil is one of the most important ingredients in Chinese perfume industry; additionally it became a prominent in the modern western perfume and fragrance industry.
1.3.2. Incense stick
Burning agarwood produces fragrance, which is used as incense for ceremonial purposes in Buddhism, Confucianism and Hinduism [11]. The incense also functions as an insect repellent [4,8]. The aromatic compounds are the main chemical components in agarwood smoke and create an atmosphere of peace and serenity [1,4]. It scent heavenly, woody nuance, balsamic and warm aura of bittersweet when the chromones break into low molecular weight at high temperature [1,4]. In Taiwan, the agarwood stick is used in traditional festivals or ceremonies to bring safety and good luck to the believer [11]. The agarwood incense stick is used in the bathroom as a customary sense, during Ramdan prayer by the Muslim, and Puja celebration by the Hindu religious practice [11].
1.3.3. Pharmaceutical use
Agarwood plays a vital role in the field of medicine, contains various chemical components, including several sesquiterpenes, 2-(2-phenylethyl)chromens (PECs), and aromatic compounds, etc [3,6,12]. These compounds display various biological properties such as anticancer, anti-inflammatory, antioxidant, antibacterial, antifungal, antidiabetic, and so on [3,6,12]. Traditionally agarwood is prescribed to treat pleurisy by the Sahih Muslim, relieve pain, arrest vomiting, and asthma [6,12]. A. malaccensis products are an essential source in the field of Ayurveda for treating various diseases such as appetizer, analgesic, antipyretic, antihistaminic, styptic, carminative, cytotoxic, insecticidal, general tonic, etc [6,11,12]. Agarwood materials have also been formulated into a balm (muscle rub) and candle wax [11]. The pharmaceutical and traditional use of agarwood in different countries/locations are presented as in Table 3.
Table 3: The pharmaceutical and traditional use of agarwood in different countries/locations.
|
Place |
Traditional use |
Preparations/route of intake |
|
Bangladesh |
Treatment of rheumatism |
Agarwood taken orally |
|
China |
Treatment of circulatory disorders, abdominal pain, vomiting, dyspnea, asthma |
Heartwood in Chinese medicines, and Heartwood decoction |
|
India |
Treatment of diarrhoea, dysentery, vomiting, anorexia, mouth and teeth diseases, facial paralysis, shivering, sprains, bone fracture |
Heartwood in Ayurvedic formulation such as Chawanprash, Arimedadi Taila and Mahanarin Taila |
|
Indonesia |
Treatment of joint pain, Sedation, detoxification, treatment of stomachaches, incense sticks |
Wood burned and smoke held over the affected area |
|
Japan |
Stomachic and sedative agent |
Infusion or decotion |
|
Korea |
Treatment of cough, acroparalysis, croup, asthma, stomachic agent, tonic, sedative and expectorant |
Infusion or decotion |
|
Malaysia |
Tonic, stimulant and carminative agent after childbirth |
Heartwood mixed with coconut oil |
|
Treatment of rheumatism and body pains |
Heartwood decoction (mixed with other types of woods) |
|
|
Treatment of small pox |
Heartwood prepared into Ointment |
|
|
Philippines |
Stop bleeding of the wounds |
Bark and roots |
|
Treatment of malaria (substitute for quinine) |
Bark, wood and fruits |
|
|
Thailand |
Treatment for diarrhoea, dysentery and skin diseases, antispasmodic and cardiovascular function enhancer in fainted patient Treatment of fainting, nausea and vomiting |
Traditional medicinal preparation ‘Krisanaglun Folk medicine ‘Ya–Hom’ |
|
Tibet |
Treatment of nervous and emotional disorders Cardioprotective agents |
Infusion or decotion |
1.3.4. Other uses
The uses of agarwood is not restricted to incense and perfumery. Solid pieces of agarwood are carved into natural art sculptures, beads, bracelets and boxes [4,11]. The wood of A. agallocha is used as decorative ornaments (China), ‘joss sticks’ (China and India), and flea and louse repellents (India), whereas the bark has been used to manufacture paper (China) [1,4]. In India, the wood of A. malaccensis used as fuel for fumigation, and the bark has been used to make cloth and rope [11].
1.4. Agarwood induction methods
Agarwood is a valuable non-timber product, and its demand is much greater than its supply. The agarwood (resin) induction mechanism is not fully understood or elucidated. High demand of quality agarwood in conjunction with the depletion of the wild Aquilaria trees, leads to the artificial induction of agarwood resin formation. Modern artificial agarwood formation techniques are mainly biochemical methods, such as chemical reagent invasion and bacteria inoculation (Figure 1) [2].
1.4.1. Natural (Traditional) methods
Naturally, agarwood formation is often linked to the physical wounding or damage of Aquilaria trees caused by thunder strike, animal grazing, pest and disease infestations [13]. These events expose the inner part of the trees toward pathogenic microbes, which evoke the defense mechanism of Aquilaria to initiate the resin production [13]. This natural formation process of agarwood has greatly inspired the development of diverse artificial induction methods (Figure 1). For example, the microbial species of Actinobacteria sp., Acidobacteria sp., Aspergillus sp., Alcaligenes sp., Bacillus sp., Chaetomium sp., Curvularia sp. Fusarium sp. Lasiodiploidia sp., Penicillium sp., Proteobacteria sp., Pseudomonas sp., and Trichoderma sp. are involved in the agarwood formation [1,13]. For more details, please refer the recent review article [221].
1.4.2. Conventional methods
Various conventional methods are applied to initiate agarwood resin formation. The techniques often involved the physical penetration into the trunk (wounding), mechanical wounding, axe chopping, nailing, holing, burning, insertion of a microbial (mainly fungal) concoction (pathology) and response of the tree towards the administered stress (non-pathological) [2,5]. Many pure-culture strains of fungi such as Aspergillus niger, A. fijiensis, Chaetomium sp., Fusarium solani, Lasiodiplodia sp. (L. hormozganensis), Gongronella butleri, Saitozyma podzolica, Cladorrhinum bulbillosum, Humicola grisea, Penicillium sp., Trichoderma lentiforme, Phaeoacremonium rubrigenum, and Tetracladium marchalianum are isolated from natural agarwood are found to be effective biological agents to induce agarwood formation in healthy Aquilaria trees [14‒16]. For more details, please refer the recent review article [221]. Therefore, fungal-interaction induction methods coupled with the application of biological inoculum are developed for agarwood induction [2,5]. The advantage of using fungal inoculum is that it is generally believed to be safe for handling and eco-friendly. However, fungal inoculation will normally give rise to localized and inconsistent quality of agarwood due to the different fungal consortium used [2,5]. As a solution, laborious holing process and long incubation time is required to maximize the colonized surface area on the tree to produce better quality of agarwood [17]. The fungal infected Aquilaria trees are reported to deposit agarwood resin around the infected sites as barrier to prevent further fungal intrusion [13,14]. Agarwood resin deposition accompanied with color changes of internal tissues occured within a year by injuring the trees [8]. Although it is cost effective and requires only personnel with little or no scientific knowledge on agarwood, but these conventional induction methods usually result in inferior quality and uncertain yield of agarwood. Mass cultivation and large plantation of Aquilaria trees using these conventional methods have greatly resolved the shortage of agarwood supply in the global market.
1.4.3. Non-conventional methods
Artificial induction of agarwood formation is the use of chemical, insect and pathogen-inducing techniques is increasingly common in agarwood induction [18]. Chemical inducers normally comprise of phytohormones, salts, minerals and biological-derived substances [2,18,19]. Various chemical induction approaches are developed, including cultivated agarwood kit (CA-kit), the whole-tree agarwood inducing technique (Agar-Wit) and biologically agarwood-inducing technique (Agar-bit). CA-kit is a combined method based on physical wounding and chemical induction, where the inducing agent is applied into the Aquilaria tree via an aeration device inserted into the wound [2]. Agar-Wit is a transpiration-assisted chemical treatment to form an overall wound in the tree, where the preloaded inducer in a transfusion set is distributed via plant transpiration [18]. Similarly, Agar-bit method adopts the idea of distributing the inducing reagent by plant transpiration, except that the reagents are injected directly into the stems of the tree [20]. Chemical inducers are suitable for mass production of agarwood with easier quality control than biological inoculum. However, in spite of the fast results and high yields, the application of chemical inducers still poses skepticism of toxicity on both human and environment. All of these induction techniques in any case mimic the natural processes of agarwood formation, which have their own strengths and weaknesses. The agarwood induction methods are presented in Figure 1. On the other hand, in vitro culture of various parts of Aquilaria spp. and Gyrinops spp. are studied at various tissue culture laboratories [2]. The tissue culture techniques identified the key regulator genes of Aquilaria spp. and Gyrinops spp. involved in the agarwood production [2].
Figure 1: Schematic presentation of Agarwood induction techniques.
2. PHYTOCHEMICAL CONSTITUENTS OF AGARWOOD
The chemical constituents of healthy Aquilaria trees without resin-formation differ from the resin-impregnated portions of the plants [6,12]. The phytochemical analysis of agarwood resin has been the subject of many studies [1,6,12]. The types and derivatives of chemical constituents in agarwood are extremely wide and diverse, indicating the different types of fragrance properties of agarwood from different species and regional sources [1,6,12]. Agarwood resin constituents were isolated using solvent extraction, with subsequent purification via column chromatography and structural elucidation using spectroscopic techniques, including NMR [1,6,12]. Essential oils are produced by the hydrodistillation of resin followed by GC-MS or the newer technique of supercritical fluid extraction (SFE) [21]. The chemical constituents in agarwood may vary considerably in terms of quality, source plant origin, extraction methods, agarwood induction method, or agarwood-formation process, collection time, analytical approach etc [1,6,12]. The agarwood chemical constituents produced by Aquilaria species including A. sinensis, A. malaccensis (syn. A. agallocha), A. crassna, A. filaria, and Gyrinops salicifolia, as well as an unidentified Aquilaria spp [1,6,12]. Previous chemical investigations of agarwood species resulted in the isolation and structure characterization of several sesquiterpenes, 2-(2-phenylethyl)-4H-chromen-4-one derivatives (PECs), and aromatic compounds are the main characteristic chemical constituents [6,12,22]. The types of agarwood chemical constituents are described below.
2.1. Sesquiterpenoids
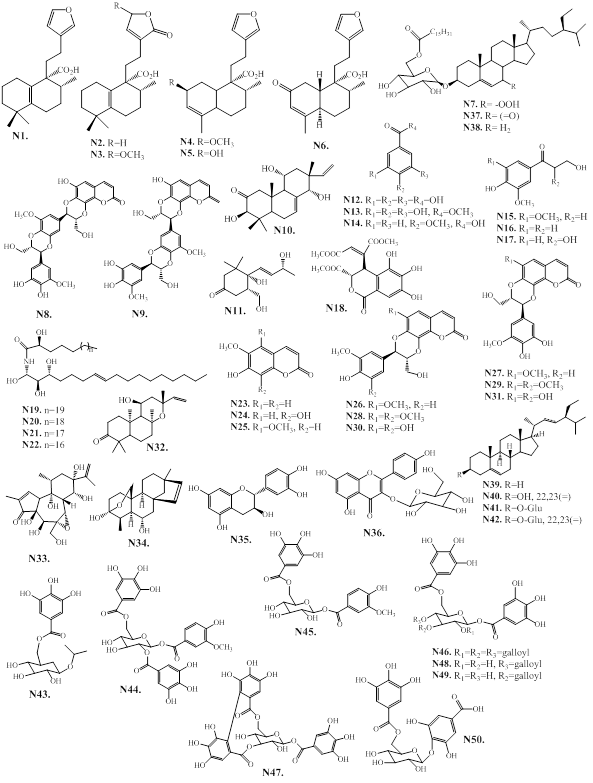
Sesquiterpenes are composed of three isoprene units. They are mainly distributed in plants existing mostly in the form of volatile constituents present in essential oils. The constituents of agarwood essential oil is mainly composed of sesquiterpenoids, and low abundant of volatile aromatic metabolites, which gives an unique and fragrant-smelling property of agarwood [1]. The sesquiterpenes isolated from agarwood exhibit various types (Figure 2), including acoranes (A), agarospiranes (B), cadinanes (C), eudesmanes (D), eremophilanes (E), guaianes (F), humulanes (G) and prezizaanes (H), zizaanes (I).
Figure 2: Types of sesquiterpenes from agarwood.
2.1.1. A. Acoranes
The spiro sesquiterpenes, acoranes (A1‒A3), are reported from the agarwood of A. sinensis (Figure 3). The compounds A2 and A3 are a pair of stereoisomers.
Figure 3: Chemical structures of acorane-type sesquiterpenes from agarwood.
2.1.2. B. Agarospiranes (vetispiranes)
The spirocyclic sesquiterpenes, agarospiranes are reported in agarwood from A. sinensis, A. malaccensis and A. agallocha (Figure 4, and Table 4). The first agarospirane sesquiterpene discovered in agarwood is agarospirol (B1) from the agarwood of A. agallocha [23]. The allyl ether 2,14-epoxy-vetispir-6-ene (B10) and enol ether 2,14-epoxy-vetispira-6(14),7-diene (B11) are reported from the essential oil of A. agallocha [24]. Vetispira-2(11),6(14)-dien-7-ol (B8) and vetispira-2(11),6-dien-14-al (B9) might be artefacts [25]. The sesquiterpenes, agarospiranes have limited distribution and are mainly found in the agarwood species of A. agallocha, A. malaccensis, and A. sinensis (Figure 4, Table 4). Phytochemical examination of 95% ethanol extract of A. agallocha agarwood, resulted in the isolation of agarospirane-type sesquiterpenes (agarospiranic aldehyde A, and B, B13, B14) [26].
Figure 4: Chemical structures of agarospirane-type sesquiterpenes from agarwood.
Table 4: Agarospirane-type sesquiterpenes from agarwood.
|
No. |
Name |
Source |
Ref. |
|
B1 |
Agarospirol |
A. agallocha A. malaccensis A. sinensis |
27,23 28 29 |
|
B2 |
Baimuxinol |
A. sinensis |
30 |
|
B3 |
Baimuxinic acid |
A. sinensis |
30 |
|
B4 |
Baimuxinal [Oxoagarospirol] |
A. sinensis A. malaccensis A. agallocha |
29, 31,32 33,34, 35 |
|
B5 |
(4R,5R,7R)-1(10)-spirovetiven-11-ol-2-one |
Kyara-Vietnam |
36 |
|
B6 |
2-Oxo-12-hydroxy-hinesol |
A. sinensis |
37 |
|
B7 |
Isoagarospirol |
25 |
|
|
B8 |
Vetispira-2(11),6(14)-dien-7-ol |
A. agallocha |
24 |
|
B9 |
Vetispira-2(11),6-dien-14-al |
A. agallocha |
24 |
|
B10 |
2,14-Epoxy-vetispir-6-ene |
A. agallocha |
24 |
|
B11 |
2,14-Epoxy-vetispira-6(14),7-diene |
A. agallocha |
24 |
|
B12 |
rel-(2R,5R,10S)-6(7)-Spirovetiven-11,12,13-triol |
Aquilaria spp. |
38 |
2.1.3. C. Cadinanes
Two (C1 and C2), decalin skeleton containing cadinane-type bicyclic sesquiterpenes are reported from agarwood of A. sinensis (C1) [39], and A. crassna (C2) [40], respectively. These two sesquiterpenes differ from eudesmane-type sesquiterpenes by the position of the isopropyl substituents and two methyl groups (Figure 5).
Figure 5: Chemical structures of cadinane-type sesquiterpenes from agarwood.
2.1.4. D. Eudesmanes (selinanes)
The main types of sesquiterpene found in agarwood are eudesmane-type sesquiterpenes, which are a class of bicyclic sesquiterpenes with a decalin skeleton. These compounds are widely distributed in the agarwood species of A. agallocha, A. crassna, A. malaccensis, and A. sinensis, as well as in G. salicifolia [12,25,416]. The eudesmane-type sesquiterpenes of agarwood are presented as Figure 6, and Table 5. Most of agarwood eudesmanes (D1–D36) contains an isopropenyl group or 2-hydroxyisopropyl group at the C-7 position, while the methyl groups at C-4 or C-11 are often oxidized to form CHO, COOH, or CH2OH groups. The eudesmanes (D3, D6, D7, D11, E19, E20 and E27) possessing an oxidation at C-9 or C-15, and an isopropenyl group at the C-7 position are reported from the acetone extract of the Vietnamese agarwood called kanankoh (A. agallocha) [35,42]. The sesquiterpenes, agarofurans, valencanes and agarospiranes (vetispiranes) biosynthetic precursor (‒)-10-epi-γ-eudesmol (D21) is isolated from A. malaccensis [33]. The nor-eudesmane derivatives D37–D40 are reported from the commercial agarwood oil (A. agallocha) [43]. The agarofuran sesquiterpenes D41–D55 has a trans-decalin structure, and a β-oriented isopropoxy bridge [12,44]. The compounds D44, D48, D49, D51, D53 and D54 are isolated from the agarwood of A. agallocha [45,46]. The sesquiterpenes D41, D43, D45, and D46 are obtained from the volatile oil of A. sinensis [47‒49]. The nor-agarofuran derivatives (D52, D54 and D55, which lack the methyl group at C-4 are only reported from agarwood of A. agallocha [43,46]. A recent study reported that the phytochemical examination of 95% ethanol extract of A. agallocha agarwood, resulted in the isolation of eudesmane-type sesquiterpenes (agalleudesmanol A-I, D56‒D64) [26]. Chemical examination of the ethyl ether extract of Aquilaria spp. collected in Thailand, resulted in the isolation and structure determination of eudesmane sesquiterpenes, D65, D66, and D67 [50].
Figure 6: Chemical structures of eudesmane-type sesquiterpenes from agarwood.
Table 5: Eudesmane-type sesquiterpenes from agarwood
|
No. |
Name |
Source |
Ref. |
|
D1. |
Agarol- [11(13)-Eudesmen-12-ol] |
A. agallocha |
51 |
|
D2. |
15-Hydroxyl-12-oxo-α-selinen |
A. sinensis |
52 |
|
D3. |
Selina-3,11-dien-14-ol |
A. agallocha |
23 |
|
D4 |
12,15-Dioxo-α-selinen [Selina-3,11-diene-12,15-dial] |
A. sinensis G. salicifolia |
32,52,53 54 |
|
D5 |
(4aβ,7β,8aβ)-3,4,4a,5,6,7,8,8a-Octahydro-7-[1-(hydroxymethyl) ethenyl]-4a-methylnaphthalene-1-carboxaldehyde |
A. malaccensis A. sinensis |
55 30,31,52 |
|
D6 |
Selina-3,11-dien-14-oic acid |
A. agallocha |
42 |
|
D7 |
(‒)-Selina-3,11-dien-14-al |
A. agallocha |
42 |
|
D8 |
(5S,7S,9S,10S)-(+)-9-hydroxy-selina-3,11-dien-12-al |
A. sinensis |
31,52 |
|
D9 |
(5S,7S,9S,10S)-(+)-9-hydroxy-eudesma-3,11(13)-dien-12-methyl ester |
A. sinensis |
31,52 |
|
D10 |
(5S,7S,9S,10S)-(‒)-9-hydroxy-selina-3,11-dien-14-al |
A. sinensis |
52 |
|
D11 |
(5S,7S,9S,10S)-(+)-selina-3,11-dien-9-ol |
A. agallocha |
35 |
|
D12 |
Petafolia A |
A. sinensis |
30 |
|
D13 |
(+)-8α-Hydroxyeudesma-3,11(13)-dien-14-al |
A. sinensis |
31 |
|
D14 |
Selina-3,11-dien-9,15-diol |
A. sinensis |
31 |
|
D15 |
(+)-Eudesma-3,11(13)-dien-8α,9β-diol |
A. sinensis |
56 |
|
D16 |
(5S,7S,10S)-(‒)-Selina-3,11-dien-9-one |
A. agallocha |
35 |
|
D17 |
Methyl-15-oxo-eudesmane-4,11(13)-dien-12-oate |
A. crassna |
57 |
|
D18 |
12,15-Dioxo-selina-4,11-dine- [Selina-4,11-diene-12,15-dial] |
A. malaccensis A. sinensis |
55 31,32 |
|
D19 |
Selina-4,11-dien-14-oic acid |
A. agallocha |
42 |
|
D20 |
Selina-4,11-dien-14-al |
A. agallocha |
42 |
|
D21 |
(‒)-10-epi-γ-eudesmol |
A. malaccensis |
33 |
|
D22 |
Eudesma-4-en-8,11-diol |
A. crassna |
57 |
|
D23 |
Eudesma-4-en-11,15-diol |
A. malaccensis A. sinensis A. crassna |
55 31 57 |
|
D24 |
12-hydroxy-4(5),11(13)-eudesmadien-15-al |
A. sinensis |
31, 30 |
|
D25 |
(7S,8R,10S)-(+)-8,12-dihydroxy-selina-4,11-dien-14-al |
A. sinensis |
52 |
|
D26 |
(+)-9β-hydroxyeudesma-4,11(13)-dien-12-al |
A. sinensis |
31 |
|
D27 |
9-hydroxy-selina-4,11-dien-14-oic acid |
A. agallocha |
42 |
|
D28 |
(7S,9S,10S)-(+)-9-hydroxy-selina-4,11-dien-14-al |
A. sinensis |
31,52,30 |
|
D29 |
(+)-eudesma-4,11(13)-dien-8α,9β-diol |
A. sinensis |
31 |
|
D30 |
(+)-eudesma-4(14),11(13)-dien-8α,9β-diol |
A. sinensis |
31 |
|
D31 |
5-desoxylongilobol |
A. sinensis A.crassna |
31 40 |
|
D32 |
Ent-4(15)-eudesmen-1α,11-diol |
A. sinensis |
52 |
|
D33 |
Eudesmane-1β,5α,11-triol |
A. sinensis |
52 |
|
D34 |
(‒)-7β-H-eudesmane-4α,11-diol |
A. sinensis |
52 |
|
D35 |
(4R,5R,7S,9S,10S)-(‒)-eudesma-11(13)-en-4,9-diol |
A. sinensis |
52 |
|
D36 |
Selin-11-en-4α-ol |
A. sinensis |
31,30 |
|
D37 |
(2R,4aS)-2-(4a-methyl-l,2,3,4,4a,5,6,7-octahydro-2-naphthyl)-propan-2-ol |
A. agallocha |
43 |
|
D38 |
(S)-4a-methyl-2-(1-methylethy1)-3,4,4a,5,6,7-hexahydronapthalene |
A. agallocha |
43 |
|
D39 |
(S)-4a-methyl-2-(1-methylethylidene)-1,2,3,4,4a,5,6,7-octahydronaphthalene |
A. agallocha |
43 |
|
D40 |
(2R,4aS)-4a-methyl-2-(1-methylethenyl)-l,2,3,4,4a,5,6,7-octahydronaphthalene |
A. agallocha |
43 |
|
D41 |
Dehydrobaimuxinol |
A. sinensis |
29,47 |
|
D42 |
4-Hydroxyl-baimuxinol |
A. sinensis |
58 |
|
D43 |
Baimuxinol |
A. sinensis |
29,47 |
|
D44 |
β-Agarofuran |
A. agallocha A. sinensis |
35,45 29,48,59 |
|
D45 |
Isobaimuxinol |
A. sinensis |
48 |
|
D46 |
Baimuxifuranic acid |
A. sinensis |
31,49 |
|
D47 |
(1S,2R,6S,9R)-6,10,10-trimethyl-ll-oxatricyclo[7.2.1.01,6]dodecane-2-carbaldehyde |
A. agallocha |
27 |
|
D48 |
4-hydroxy-dihydro-agarofuran |
A. agallocha |
46 |
|
D49 |
α-Agarofuran |
A. agallocha A. malaccensis |
45 33 |
|
D50 |
Epoxy-β-agarofuran |
A. agallocha |
27 |
|
D51 |
Dihydro-β-agarofuran |
A. agallocha |
45 |
|
D52 |
(1R,6S,9R)-6,10,10-trimethyl-11-oxatricyclo[7.2.1.0]dodecane |
A. agallocha |
43 |
|
D53 |
3,4-dihydroxy-dihydro-agarofuran |
A. agallocha |
46 |
|
D54 |
Nor-ketoagarofuran |
A. agallocha |
46 |
|
D55 |
(1R,2R,6S,9R)-6,10,10-trimethyl-11-oxatricyclo[7.2.1.0]dodecan-2-ol |
A. agallocha |
43 |
|
D56 |
Agalleudesmanol A |
A. agallocha |
26 |
|
D57 |
Agalleudesmanol B |
A. agallocha |
26 |
|
D58 |
Agalleudesmanol C |
A. agallocha |
26 |
|
D59 |
Agalleudesmanol D |
A. agallocha |
26 |
|
D60 |
Agalleudesmanol E |
A. agallocha |
26 |
|
D61 |
Agalleudesmanol F |
A. agallocha |
26 |
|
D62 |
Agalleudesmanol G |
A. agallocha |
26 |
|
D63 |
Agalleudesmanol H |
A. agallocha |
26 |
|
D64 |
Agalleudesmanol I |
A. agallocha |
26 |
|
D65 |
5β,7β-H-elema-1,3-dien-11,13-dihydroxy-11-methyl ester |
Aquilaria sp. |
50 |
|
D66 |
5β,7β-H-4α-hydroxy-eudesma-11,13-dihydroxy-11-methyl ester |
Aquilaria sp. |
50 |
|
D67 |
5α,7α-H-4(14)-ene-eudesma-11,13- dihydroxy-11-methyl ester |
Aquilaria sp. |
50 |
2.1.5. E. Eremophilanes (valencanes)
The chemical structures of eremophilane-type sesquiterpenes from agarwood consist two six-membered rings (E1 to E38) are presented in Figure 7, and Table 6. The reported eremophilanes contain a tri-oxygenated isopropyl group (E6, E14, E23, E24, E29, E33, and E34), and an 11-methyl ester functionality (E24, E29, and E34). The G. salicifolia compound, rel-4b,5b,7b-eremophil-9-en-12,8a-olide (E11) is the only one of an eremophilane containing an 8,12-lactone ring [60]. The A. agallocha essential oils compound E36 exhibits a nor-skeleton of eremophilane [27], which might be a degradation product of major agarwood compound, dihydrokaranone (E25). The eremophilanes, (+)-(4S,5R)-karanone (E22) and (+)-(4S,5R)-dihydrokaranone (E25) are unsaturated and conjugated ketones. These two compounds present in most of the essentials and extracts of Aquilaria species, except for A. malaccensis from Indonesia, and are characteristic constituents of agarwood [25]. The compounds E16 and E26 are a pair of epimers at C-7, and have strong long-lasting pennyroyal-like minty smell [58]. Chemical examination of the ethyl ether extract of Aquilaria sp. collected in Thailand, resulted in the isolation and structure determination of eremophilane sesquiterpenes, E40, and E41 [50].
Figure 7: Chemical structures of eremophilane-type sesquiterpenes from agarwood.
Table 6: Eremophilane-type sesquiterpenes of agarwood.
|
No. |
Name |
Source |
Ref. |
|
E1. |
Eremophila-9,11(13)-dien-12-ol |
A. agallocha |
24 |
|
E2. |
Valenc- or eremophil-9-en-12-al (tentative) |
A. agallocha |
24 |
|
E3. |
Jinkoh-eremol |
A. malaccensis |
28 |
|
E4 |
(1β,3α,4aβ,5β,8aα)-4,4a-dimethyl-6(prop-l-en-2-yl)octahydronaphtha-lene-1,8a(1H)-diol |
A. crassna |
57 |
|
E5 |
(1aβ,2β,3β,4aβ,5β,8aβ)-octahydro-4a,5-dimethyl-3-(1-methylethenyl)-3H-naphth[1,8a-b]oxiren-2-ol |
A. malaccensis |
55 |
|
E6 |
Eremophil-9-ene-11,12,13-triol |
Aquilaria spp. |
38 |
|
E7 |
(+)-9β,10β-epoxyeremophila-11(13)-en |
A. sinensis |
31 |
|
E8 |
(1β,4aβ,7β,8aβ)-octahydro-7-[1-(hydroxymethyl)ethenyl]-1,8a-dimethylnaphthalen-4a(2H)-ol |
A. malaccensis A. sinensis |
55 31,61 |
|
E9 |
2-[(2β,4aβ,8β,8aβ)-decahydro-4α-hydroxy-8,8a-dimethylnaphthalen-2-yl]prop-2-enal |
A. malaccensis A. sinensis |
55 31 |
|
E10 |
11,13-dihydroxy-9(10)-ene-8β,12-epoxyemophilane |
A. crassna Aquilaria spp. |
40 38 |
|
E11 |
rel-4β,5β,7β-eremophil-9-en-12,8α-olide |
G. salicifolia |
60 |
|
E12 |
8,12-epoxy-eremophila-9,11(13)-diene |
A. agallocha |
24 |
|
E13 |
Eremophil-9(10)-ene-11,12-diol |
G. salicifolia |
41 |
|
E14 |
4β,7α-H-eremophil-9(10)-ene-11,12,13-triol |
G. salicifolia |
41 |
|
E15 |
4β,7α-H-eremophil-9(10)-ene-12,13-diol |
G. salicifolia |
41 |
|
E16 |
7β-H-9(10)-ene-11,12-epoxy-8-oxoeremophilane |
A. sinensis |
58 |
|
E17 |
Ligudicin C |
A. sinensis |
53, 62 |
|
E18 |
(‒)-Eremophila-9-en-8β,11-diol |
A. sinensis A. crassna |
31 57 |
|
E19 |
4β,7α,8α-H-eremophil-9(10)-ene-8,12-epoxy-11α,13-diol |
G. salicifolia |
41 |
|
E20 |
Cyclodebneyol |
A. sinensis |
37 |
|
E21 |
Dehydro-jinkoh-eremol |
A. agallocha |
42 |
|
E22 |
(+)-(4S,5R)-Karanone |
A. agallocha |
35 |
|
E23 |
4β,7α-H-eremophil-1(2),9(10)-dien-11,12,13-triol |
G. salicifolia |
41 |
|
E24 |
4β,7α-H-11,13-dihydroxy-eremophil-1(10)-ene-11-methyl ester |
G. salicifolia |
41 |
|
E25 |
(+)-(4S,5R)-dihydrokaranone- [7(11)-eremophilen-8-one] |
A. sinensis A. agallocha |
30,53,59,29,62 35,53 |
|
E26 |
7α-H-9(10)-ene-11,12-epoxy-8-oxoeremophilane |
A. sinensis A. crassna |
58,61,62 40 |
|
E27 |
Petafolia B |
A. sinensis |
30 |
|
E28 |
Neopetasane- [Eremophila-9,11-dien-8-one] |
A. agallocha A. malaccensis A. sinensis |
42 34 30,53,58,61,62 |
|
E29 |
(4S,5R,7R)-11,12-dihydroxy-eremophila-1(10)-ene-2-oxo-11-methylester |
A. crassna |
62 |
|
E30 |
Kusunol- [Valerianol] |
A. malaccensis A. agallocha A. sinensis |
24 25 29,61 |
|
E31 |
2-[(2β,8α,8aα)-8,8a-dimethyl-1,2,3,4,6,7,8,8a-octahydronaphthalen-2-yl]propane-1,2-diol |
A. crassna |
57 |
|
E32 |
(+)-trans-Nootkatol |
G. salicifolia |
41 |
|
E33 |
2-[(2β,8β,8aα)-8,8a-dimethyl-1,2,3,4,6,7,8,8a-octahydronaphthalen-2-yl]-3-hydroxy-2-methoxypropanoic acid |
A. crassna |
57 |
|
E34 |
Methyl crassicid |
A. crassna |
63 |
|
E35 |
Valenca-1(10),8-dien-11-ol |
A. agallocha |
24 |
|
E36 |
2,3-dimethyl-r-2-(3-methyl-2-butenyl)-1-cyclohexanone |
A. agallocha |
27 |
|
E37 |
11-hydroxy-valenc-1(l0)-en-2-one |
A. sinensis |
30,31,61 |
|
E38 |
(+)-11-hydroxyvalenc-1(10),8-dien-2-one |
A. sinensis |
31 |
|
E39 |
Valencene |
A. malaccensis |
64 |
|
E40 |
7β-H-9(10)-ene-emophane-11,13-dihydroxy-11-methyl ester |
Aquilaria sp. |
50 |
|
E41 |
7α-H-11α,13-dihydroxy-9(10)-ene-8α,12-epoxyemophane |
Aquilaria sp. |
50 |
2.1.6. F. Guaianes
The sesquiterpene guaianes are structurally coupled with a five- and seven-membered ring structures, and are consisting of a 4,10-dimethyl-7-isopropenyl moiety. The isolated and structure identified guaianes (F1–F47) from the species of Aquilaria and Gyrinops are presented as in Figure 8, and Table 7. The guaianes F2–F11, and F13 bearing a 7-isopropenyl moiety are considered as the characteristic components from the agarwood of A. agallocha, namely kanankoh. The characteristic compound of kanankoh, (‒)-guaia-1(10),11-dien-15-al (F7) has a pleasant β-damascenone-like woody and floral note with a slight cooling side note [35,36]. Among the kanankoh compounds, the isolates F3, F4, F6, F7, F10 and F11 are functionalized at C-14, which is rarely encountered in nature. The compound, (+)-1,5-epoxy-nor-ketoguaiene (F13) is a nor-guaiane with 14 carbons lacking the methyl group at C10. On the other hand, the tricyclic scaffold patchoulenetype compounds F14–F16 are isolated from the agarwood of A. malaccensis [66]. The A. sinensis agarwood compound F19 possesses a 5/6/7 ring system of guaiane skeleton through C1–C11 linkage. It is interesting to note that the agarwood species A. sinensis is a rich source for various interesting chemical structures. The compounds F17–F31 and F33 are reported from the agarwood of A. sinensis [59]. The guaiane-furans (F20–F25) are reported from a agarwood variety of A. sinensis, namely “Lv Qi-Nan” in Chinese [67]. These compounds possess a 5,11-epoxy ring with stereoisomers, and are functionalized at C15 (Figure 8, Table 7). Furthermore, the guaianes F33 and F34, with cleavage of the seven-membered core ring also obtained from the agarwood of A. sinensis [32]. Additionally, the guaianes, F28, F32 and F38, which are possessing a bridge in the seven-membered ring structure are also reported from the agarwood of A. sinensis[61,68]. Among the guaiane sesquiterpenes bearing five-membered lactone, the compounds F35‒F37 and F41 are reported from the agarwood of A. filaria and G. salicifolia. These compounds have typical conjugated double bonds within the seven-membered ring, as well as a five-membered α,β-unsaturated lactone [41,68]. Phytochemical examination of A. malaccensis resulted in the isolation of guaiane-type sesquiterpenes F43‒F47 [69].
Figure 8: Chemical structures of guaiane-type sesquiterpenes from agarwood.
Table 7: Guaiane-type sesquiterpenes isolated from agarwood species.
|
No. |
Name |
Source |
Ref. |
|
F1. |
(+)-Guaia-1(10),11-dien-9-one |
A. agallocha |
55 |
|
F2. |
(‒)-1,10-epoxyguai-11-ene |
A. agallocha |
55 |
|
F3. |
Methyl guaia-1(10),11-diene-15-carboxylate |
A. agallocha |
35, 55 |
|
F4 |
(‒)-Guaia-1(10),11-diene-15-carboxylic acid |
A. agallocha |
55 |
|
F5 |
α-Bulnesene |
A. agallocha |
35 |
|
F6 |
(‒)-Guaia-1(10),11-dien-15-ol |
A. agallocha |
55 |
|
F7 |
(‒)-Guaia-1(10),11-dien-15-al |
A. agallocha |
35, 55 |
|
F8 |
α-Guaiene |
A. agallocha |
35 |
|
F9 |
(‒)-Rotundone |
A. agallocha |
55 |
|
F10 |
(‒)-Guaia-1(10),11-dien-15,2-olide |
A. agallocha |
55 |
|
F11 |
(‒)-2α-hydroxyguaia-1(10),11-dien-15-oic acid |
A. agallocha |
70 |
|
F12 |
(+)-12,13-dihydroxyguaiol |
Aquilaria spp. |
38 |
|
F13 |
(+)-1,5-epoxy-nor-ketoguaiene |
A. agallocha |
42 |
|
F14 |
Auranticanol A |
A. malaccensis |
66 |
|
F15 |
Chamaejasmone D |
A. malaccensis |
66 |
|
F16 |
Chamaejasmone E |
A. malaccensis |
66 |
|
F17 |
α-Kessyl alcohol |
A. sinensis |
71 |
|
F18 |
Epi-guaidiol A |
A. sinensis |
37 |
|
F19 |
Qinan-guaiane-one |
A. sinensis |
71 |
|
F20 |
Qinanol E |
A. sinensis |
67 |
|
F21 |
Qinanol C |
A. sinensis |
67 |
|
F22 |
Qinanol A |
A. sinensis |
67 |
|
F23 |
Qinanol B |
A. sinensis |
67 |
|
F24 |
Qinanol D |
A. sinensis |
67 |
|
F25 |
Sinenofuranal |
A. sinensis |
59 |
|
F26 |
Sinenofuranol |
A. sinensis |
59, 67 |
|
F27 |
1,5;8,12-diepoxyguaia-12-one |
A. sinensis |
61 |
|
F28 |
3-Oxo-7-hydroxylholosericin A |
A. sinensis |
61 |
|
F29 |
1α-hydroxy-4α,10α-dimethyl-5βH-octahydro-azulen-8-one |
A. sinensis |
32 |
|
F30 |
Qinanlactone |
A. sinensis |
71 |
|
F31 |
7βH-Guaia-1(10)-en-12,8β-olide |
A. sinensis |
32 |
|
F32 |
1,8-Epoxy-5H-guaia-9-en-12,8-olide |
A. filaria |
68 |
|
F33 |
1,10-dioxo-4αH-5αH-7βH-11αH-1,10-secoguaia-2(3)-en-12,8β-olide |
A. sinensis |
32 |
|
F34 |
1α-hydroxy-4βH-5βH-7βH-11αH-8,9-secoguaia-9(10)-en-8,12-olide |
A. sinensis |
32 |
|
F35 |
2-Oxoguaia-1(10),3,5,7(11),8-pentaen-12,8-olide |
G. salicifolia A. filaria |
41 68 |
|
F36 |
(4R,5S)-3-Oxo-5,6-dihydro-gweicurculactone |
A. filaria |
68 |
|
F37 |
(4R)-3-Oxo-gweicurculactone |
A. filaria |
68 |
|
F38 |
1(5)-Ene-7,10-epoxy-guaia-12-one |
A. filaria |
68 |
|
F39 |
Guaianolide |
G. salicifolia A. filaria |
41 68 |
|
F40 |
4β,5α,7α,8α-H-3β-hydroxy-1(10)-ene-8,12-epoxy-guaia-12-one |
G. salicifolia |
41 |
|
F41 |
(−)-Gweicurculactone |
G. salicifolia |
41 |
|
F42 |
Aromadendrene |
A. malaccensis |
72 |
|
F43 |
2-Oxo-5β,10β-peroxyl-1αH,4αH,7αH,8βH-guaian-8α,12-olide |
A. malaccensis |
69 |
|
F44 |
10α-hydroxy-4αH,5αH,7αH,8βH-guaia-1(2)-en-8α,12-olide |
A. malaccensis |
69 |
|
F45 |
4αH,7αH-14-nor-guaia-1(5)-en-8α,12-olide |
A. malaccensis |
69 |
|
F46 |
1α,7α-dihydroxy-8oxo-4αH,5αH-guaia-9(10),11(13)-dien-12-oate |
A. malaccensis |
69 |
|
F47 |
7β,10β-epoxy-4αH-guaia-1(5),11(13)-dien-12-ol |
A. malaccensis |
69 |
2.1.7. G. Humulanes
Four humulane-type sesquiterpenes (G1–G4) are reported from the agarwood of A. sinensis and A. malaccensis (Figure 9) [31,66]. The compounds, quilanols A and B (G1 and G2) possess an unprecedented macrocyclic humulene structure with a bicyclic 7/10 ring system [66]. The sesquiterpene β-caryophyllene (G5) is reported from from the essential oil of A. crassna [73]. Phytochemical examination of A. malaccensis resulted in the isolation of humulene-type sesquiterpenes G6‒G9 [69].
Figure 9: Chemical structures of humulane-type sesquiterpenes from agarwood.
2.1.9. H. Prezizaanes
The tricyclic prezizaanetype sesquiterpenes jinkohol II (H1) and jinkohol (H11) are reported from the agarwood of A. malaccensis [25,28,74]. Then the prezizaane-type sesquiterpenes (H1–H17), are reported from the agarwood of Aquilaria spp. collected in Thailand (Figure 10 and Table 8).
Figure 10: Chemical structures of prezizaane-type sesquiterpenes from agarwood.
Table 8: Prezizaane-type sesquiterpenes from agarwood.
|
No. |
Name |
Source |
Ref. |
|
H1. |
Jinkohol Ⅱ |
A. malaccensis Aquilaria spp. |
28 75 |
|
H2. |
Jinkoholic acid |
Aquilaria spp. |
75 |
|
H3. |
Aquilarene E |
Aquilaria spp. |
76 |
|
H4 |
Aquilarene D |
Aquilaria spp. |
76 |
|
H5 |
Agarozizanol B |
Aquilaria spp. |
75 |
|
H6 |
Agarozizanol C |
Aquilaria spp. |
75 |
|
H7 |
Aquilarene C |
Aquilaria spp. |
76 |
|
H8 |
Agarozizanol D |
Aquilaria spp. |
75 |
|
H9 |
Aquilarene B |
Aquilaria spp. |
76 |
|
H10 |
Aquilarene A |
Aquilaria spp. |
76 |
|
H11 |
Jinkohol |
A. malaccensis Aquilaria spp. |
74 75 |
|
H12 |
Aquilarene F |
Aquilaria spp. |
76 |
|
H13 |
Aquilarene G |
Aquilaria spp. |
76 |
|
H14 |
Agarozizanol A |
Aquilaria spp. |
75 |
|
H15 |
Aquilarene I |
Aquilaria spp. |
76 |
|
H16 |
Aquilarene H |
Aquilaria spp. |
76 |
|
H17 |
Aquilarene J |
Aquilaria spp. |
76 |
2.1.10. I. Zizaanes
Three tricyclic sesquiterpenes of the zizaane skeleton (I1–I3) are reported from agarwood of Aquilaria spp., collected from Thailand (Figure 11) [75].
Figure 11: Chemical structures of zizaane-type sesquiterpenes from agarwood.
2.1.11. J. Other sesquiterpenoids
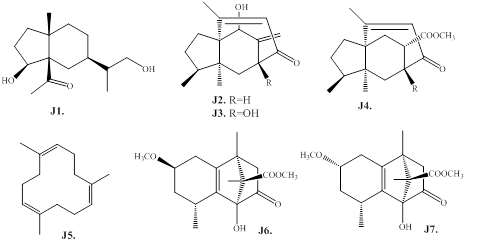
In addition to the aforementioned sesquiterpenoids, the species of the agarwood also resulted in the isolation and structure determination different minor sesquiterpenes (Figure 12, Table 9). For example, the eudesmane skeleton compound, 12-hydroxy-dihydrocyperolone (J1) is obtained as a new one from the agarwood of G. salicifolia [60]. The daphnauranols B–D (J2–J4) exhibiting a rare 5/6/7 ring system were obtained from the agarwood of A. malaccensis [66]. Furthermore, the agarwood of A. malaccensis also resulted in the isolation and chemical structure identification of tricyclic cadinene-rearranged-sesquiterpenoids with a 6/6/5 ring system, malacinones A and B (J6 and J7) [77]. On the other hand, the compound 1,5,9-trimethyl- 1,5,9-cyclododecatriene (J5) is obtained from the from the agarwood of A. sinensis [61].
Figure 12: Chemical structures of other sesquiterpenes from agarwood.
Table 9: Other sesquiterpenes from agarwood.
|
No. |
Name |
Source |
Ref. |
|
J1 |
12-Hydroxy-dihydrocyperolone |
G. salicifolia |
60 |
|
J2 |
Daphnauranol C |
A. malaccensis |
66 |
|
J3 |
Daphnauranol B |
A. malaccensis |
66 |
|
J4 |
Daphnauranol D |
A. malaccensis |
66 |
|
J5 |
1,5,9-Trimethyl-1,5,9-cyclododecatriene |
A. sinensis |
61 |
|
J6 |
Malacinone B |
A. malaccensis |
77 |
|
J7 |
Malacinone A |
A. malaccensis |
77 |
All these isolation and structure identification reports indicating that agarwood is a rich source for various sesquiterpenes including, acorane, agarospirane, cadinane, eudesmane, eremophilane, guaiane, humulane, prezizaane, or zizaane, etc. Among the reported sesquiterpenes of agarwood, eremophilanes, eudesmanes, and guaianes are widely distributed in various agarwood species. Most of these sesquiterpenes are reported from the agarwood species of A. agallocha, A. crassna, A. malaccensis, and A. sinensis. Additionally, these sesquiterpenes also reported from the other species of agarwood including A. filaria, G. salicifolia, and an unidentified Aquilaria spp.
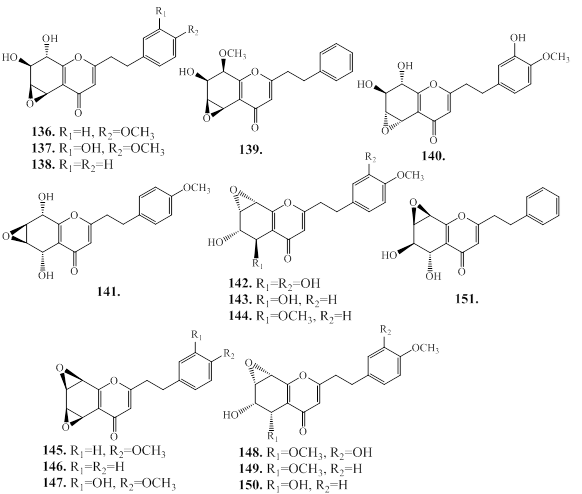
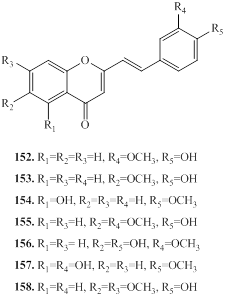
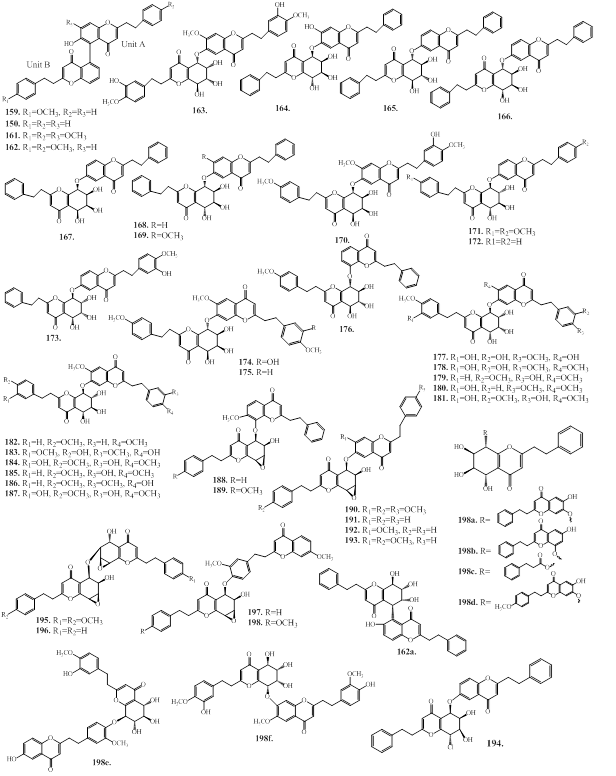
2.2. 2-(2-phenylethyl)chromones (PECs)
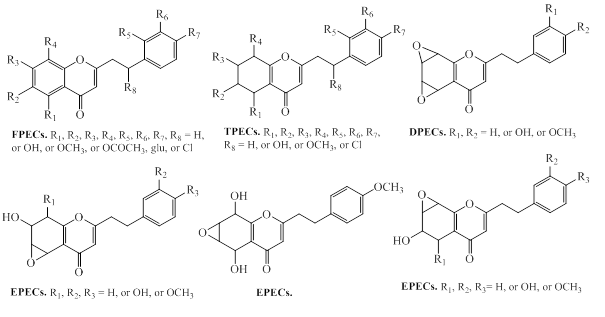
2-(2-phenylethyl)chromones (PECs) is a member of the class of chromones, which are substituted by a 2-phenylethyl group at C2 position [78]. These compounds has structural resembling with flavonoids, which bears only phenyl group at C-2 position, instead of 2-phenylethyl group present in PECs [78]. PEC derivatives are other major group of constituents in agarwood species [6,12]. The PECs are responsible for the fragrances odor of agarwood burning or heating [12]. The natural PECs are reported from plant species of Eremophila georgei, Bothriochloa ischaemum (Gramineae), and agarwood of Aquilaria spp [6,12]. Depending on the molecular skeleton, PECs are mainly divided into monomeric 2-(2-phenylethyl)chromone, dimeric 2-(2-phenylethyl)chromones, sesquiterpenoid-4H-chromones and benzylacetone-4H-chromones, and trimeric chromones as described below.
2.2.1. Monomeric 2-(2-phenylethyl)chromone
Following the characteristic structure of the chromone skeleton, monemeric PECs are subdivided into four groups as Flindersia type 2-(2-phenylethyl)chromones (FPECs), 5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones (TPECs), mono-epoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones (EPECs), and diepoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones (DPECs) (Figure 13).
Figure 13: Chemical structures of monomeric 2-(2-phenylethyl)chromones types of agarwood.
2.2.1a. Flindersia type 2-(2-phenylethyl)chromones (FPECs)
The FPECs are the most abundant PECs in agarwood species (1–86). Additionally, a new FPEC (85a) is reported from the MeOH extract of agarwood Jink [79]. The chemical structures of FPECs are presented in Figure 14, and their natural source in Table 10.
Figure 14: Skeleton of Flindersia type 2-(2-phenylethyl)chromones from agarwood.
Table 10: Flindersia type 2-(2-phenylethyl)chromones from agarwood species.
|
No. |
Name |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Source |
Ref. |
|
1 |
2-[2-(4-hydroxyphenyl)ethyl]chromone [Qinanone D] |
H |
H |
H |
H |
H |
H |
OH |
H |
A. sinensis |
80,81 |
|
2 |
2-[2-(3-hydroxyphenyl)ethyl]chromone [Qinanone E] |
H |
H |
H |
H |
H |
OH |
H |
H |
A. sinensis |
80 |
|
3 |
2-[2-(2-hydroxyphenyl)ethyl]chromone [Qinanone F] |
H |
H |
H |
H |
OH |
H |
H |
H |
A. sinensis A. malaccensis |
80 82 |
|
4 |
8-hydroxy-2-(2-phenylethyl)chromone |
H |
H |
H |
OH |
H |
H |
H |
H |
A. sinensis A. filaria A. malaccensis |
83 84 82 |
|
5 |
7-hydroxy-2-(2-phenylethyl)chromone |
H |
H |
OH |
H |
H |
H |
H |
H |
A. malaccensis |
78 |
|
6 |
6-hydroxy-2-(2-phenylethyl)chromone |
H |
OH |
H |
H |
H |
H |
H |
H |
Kalimantan A. sinensis A. malaccensis A. filaria G. crassna Aquilaria spp. |
86 53,80,87,88 34,82 84 89 90 |
|
7 |
5-hydroxy-2-(2-phenylethyl)chromone |
OH |
H |
H |
H |
H |
H |
H |
H |
A. sinensis A. malaccensis |
91 82 |
|
8 |
(S)-2-(2-hydroxy-2-phenylethyl)chromone |
H |
H |
H |
H |
H |
H |
H |
S-OH |
A. crassna A. filaria |
89 84 |
|
9 |
(R)-2-(2-hydroxy-2-phenylethyl)chromone |
H |
H |
H |
H |
H |
H |
H |
R-OH |
A. crassna A. sinensis A. filaria |
89 92,93 84 |
|
10 |
2-(2-phenylethyl)chromone [flidersiachromone] |
H |
H |
H |
H |
H |
H |
H |
H |
Vietnam A. agallocha A. sinensis A. malaccensis A. filaria Aquilaria spp. |
94 35 37,62,80,91,92,95 34,82,85 68,84 90 |
|
11 |
7-methoxy-2-(2-phenylethyl)-4H-chromen-4-one |
H |
H |
OCH3 |
H |
H |
H |
H |
H |
A. malaccensis A. sinensis |
82,96 53,62,91 |
|
12 |
6-methoxy-2-(2-phenylethyl)chromone [AH4] |
H |
OCH3 |
H |
H |
H |
H |
H |
H |
Kalimantan A. sinensis A. agallocha A. malaccensis Aquilaria spp. |
86 29,95 97 34,82 90 |
|
13 |
2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
H |
H |
H |
H |
H |
OCH3 |
H |
Vietnam A. agallocha A. malaccensis A. sinensis |
94 35,98 82,96 80,91,99 |
|
14 |
5,8-dihydroxy-2-(2-phenylethyl)chromone [AH7] |
OH |
H |
H |
OH |
H |
H |
H |
H |
Kalinantan A. sinensis |
100 91,99,101 |
|
15 |
5-hydroxy-2-[2-(2-hydroxyphenyl)ethyl]chromone |
OH |
H |
H |
H |
OH |
H |
H |
H |
A. crassna |
102 |
|
16 |
5,6-dihydroxy-2-(2-phenylethyl)chromone |
OH |
OH |
H |
H |
H |
H |
H |
H |
A. crassna A. malaccensis |
103 82 |
|
17 |
6-hydroxy-2-[2-(4-hydroxyphenyl)ethyl]chromone |
H |
OH |
H |
H |
H |
H |
OH |
H |
A. malaccensis A. sinensis A. filaria G. salicifolia A. crassna |
85 81,87 84 54 102 |
|
18 |
6-hydroxy-2-[2-(2-hydroxyphenyl)ethyl]chromone |
H |
OH |
H |
H |
OH |
H |
H |
H |
A. malaccensis A. sinensis |
85 80,92,87 |
|
19 |
6,7-dihydroxy -2-(2-phenylethyl)chromone |
H |
OH |
OH |
H |
H |
H |
H |
H |
G. salicifolia Aquilaria spp. A. sinensis Jinko |
54 104 37 79 |
|
20 |
6,8-dihydroxy-2-(2-phenylethyl)chromone |
H |
OH |
H |
OH |
H |
H |
H |
H |
A. malaccensis A. sinensis A. filaria Aquilaria spp. |
85 88,92,99 84 104 |
|
21 |
2-[2-hydroxy-2-(4-hydroxyphenyl)ethyl]chromone |
H |
H |
H |
H |
H |
H |
OH |
OH |
A. sinensis |
83 |
|
22 |
6-hydroxy-2-(2-hydroxy-2-phenylethyl)chromone |
H |
OH |
H |
H |
H |
H |
H |
OH |
A. sinensis |
95, 93 |
|
23 |
6-methoxy-7-hydroxy-2-(2-phenylethyl) chromone |
H |
OCH3 |
OH |
H |
H |
H |
H |
H |
A. sinensis |
93,105 |
|
24 |
6-hydroxy-5-methoxy-2-(2-phenylethyl)chromone |
OCH3 |
OH |
H |
H |
H |
H |
H |
H |
A. sinensis |
62 |
|
25 |
5-hydroxy-6-methoxy-2-(2-phenylethyl)chromone |
OH |
OCH3 |
H |
H |
H |
H |
H |
H |
A. sinensis A. malaccensis |
95 82,96 |
|
26 |
6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone |
H |
OH |
OCH3 |
H |
H |
H |
H |
H |
A. malaccensis A. sinensis A. filaria |
85 53,62 84 |
|
27 |
6-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
OH |
H |
H |
H |
H |
OCH3 |
H |
A. sinensis A. crassna A. filarial A. malaccensis |
80,81,88,99 106 84 82 |
|
28 |
6-methoxy-2-[2-(4-hydroxyphenyl)ethyl]chromone [Aquilarone H] |
H |
OCH3 |
H |
H |
H |
H |
OH |
H |
A. sinensis |
88,107 |
|
29 |
6-methoxy-8-hydroxy-2-(2-phenylethyl) chromone |
H |
OCH3 |
H |
OH |
H |
H |
H |
H |
A. crassna |
108 |
|
30 |
6-methoxy-2-[2-(2-hydroxyphenyl)ethyl]chromone |
H |
OCH3 |
H |
H |
OH |
H |
H |
H |
A. crassna |
103 |
|
31 |
6-methoxy-2-[2-(3-hydroxyphenyl)ethyl]chromone |
H |
OCH3 |
H |
H |
H |
OH |
H |
H |
A. sinensis |
88,107 |
|
32 |
2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone [Qinanone A] |
H |
H |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis |
80 |
|
33 |
2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone [Qinanone B] |
H |
H |
H |
H |
H |
OCH3 |
OH |
H |
A. sinensis A. crassna |
80,81 89 |
|
34 |
7-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
H |
OH |
H |
H |
H |
OCH3 |
H |
A. sinensis |
62 |
|
35 |
2-[2-(2-hydroxy-4-methoxyphenyl)ethyl]chromone [Qinanone C] |
H |
H |
H |
H |
OH |
H |
OCH3 |
H |
A. sinensis |
80 |
|
36 |
7-methoxy-2-[2-(4-hydroxyphenyl)ethyl]chromone |
H |
H |
OCH3 |
H |
H |
H |
OH |
H |
A. sinensis A. crassna |
62 109 |
|
37 |
6-hydroxy-8-chloro-2-(2-phenylethyl)chromone |
H |
OH |
H |
Cl |
H |
H |
H |
H |
A. sinensis A. filaria A. crassna A. malaccensis |
91,93,110 84 63 111 |
|
38 |
2-[2-hydroxy -2-(4-methoxyphenyl)ethyl]chromone |
H |
H |
H |
H |
H |
H |
OCH3 |
OH |
A. sinensis A. crassna |
90 89 |
|
39 |
5,8-dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
OH |
H |
H |
OH |
H |
H |
OCH3 |
H |
A. sinensis G. salicifolia |
105 111 |
|
40 |
6,7-dimethoxy-2-(2-phenylethyl)chromone [AH6] |
H |
OCH3 |
OCH3 |
H |
H |
H |
H |
H |
Kalinantan A. sinensis A. agallocha Kyara 1st (Vietnam) A. malaccensis A. crassna A. filaria Aquilaria spp. |
86 53,62,112 88,92,95 83,105 97 36 34,82 106 84 90 |
|
41 |
5,8-dihydroxy-6-methoxy-2-(2-phenylethyl)chromone |
OH |
OCH3 |
H |
OH |
H |
H |
H |
H |
A. sinensis |
113 |
|
42 |
6-methoxy-2-[2-(3-methoxyphenyl)ethyl]chromone [AH5] |
H |
OCH3 |
H |
H |
H |
OCH3 |
H |
H |
Kalimantan A. sinensis A. malaccensis |
86 53,112,95 82,96 |
|
43 |
2-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
OCH3 |
H |
H |
H |
H |
OCH3 |
H |
A. agallocha A. sinensis A. malaccensis |
35,98 29,112,88 96 |
|
44 |
6,8-dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
OH |
H |
OH |
H |
H |
OCH3 |
H |
A. sinensis Aquilaria spp. |
99,114 104 |
|
45 |
6-hydroxy-2-[2-(3-hydroxy-4- methoxyphenyl)ethyl]chromone [Aquilarone I] |
H |
OH |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis Aquilaria spp. |
80,81,93,107 90 |
|
46 |
6-hydroxy-7-methoxy-2-[2-(4-hydrxoyphenyl)ethyl] chromone |
H |
OH |
OCH3 |
H |
H |
H |
OH |
H |
A. sinensis G. salicifolia Aquilaria spp. |
93,115 111 116 |
|
47 |
6-hydroxy-2-[2-(3-methoxy-4- hydroxyphenyl)ethyl]chromone |
H |
OH |
H |
H |
H |
OCH3 |
OH |
H |
A. sinensis Aquilaria spp. Aquilaria spp. |
80,93,114,117 104 75 |
|
48 |
6,7-dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
OH |
OH |
H |
H |
H |
OCH3 |
H |
A. sinensis G. salicifolia Aquilaria spp. |
114,115 54 104 |
|
49 |
5-hydroxy-8-methoxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
OH |
H |
H |
OCH3 |
H |
H |
OCH3 |
H |
A. sinensis |
99 |
|
50 |
5-hydroxy-6-methoxy-2-[2-(3-methoxyphenyl)ethyl] Chromone |
OH |
OCH3 |
H |
H |
H |
OCH3 |
H |
H |
A. sinensis |
53 |
|
51 |
5-hydroxy-7-methoxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
OH |
H |
OCH3 |
H |
H |
H |
OCH3 |
H |
A. sinensis |
113 |
|
52 |
6-hydroxy-5-methoxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
OCH3 |
OH |
H |
H |
H |
H |
OCH3 |
H |
A. sinensis |
53 |
|
53 |
6-hydroxy-8-chloro-2-[2-(4-hydroxyphenyl)ethyl]chromone |
H |
OH |
H |
Cl |
H |
H |
OH |
H |
Aquilaria spp. |
116 |
|
54 |
5-Hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
OH |
OCH3 |
H |
H |
H |
H |
OCH3 |
H |
A. malaccensis A. sinensis |
96 88 |
|
55 |
6,7-dimethoxy-2-[2-(3-hydroxyphenyl)-ethyl]chromone |
H |
OCH3 |
OCH3 |
H |
H |
OH |
H |
H |
A. sinensis |
81 |
|
56 |
6-methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl] chromone |
H |
OCH3 |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis A. crassna Aquilaria spp. |
88 106 90 |
|
57 |
6-methoxy-7-hydroxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
H |
OCH3 |
OH |
H |
H |
H |
OCH3 |
H |
A. malaccensis A. sinensis A. crassna |
34 81,99,114 102,108 |
|
58 |
6-hydroxy-2-[2-(3,4-dimethoxyphenyl)ethyl]chromone |
H |
OH |
H |
H |
H |
OCH3 |
OCH3 |
H |
A. sinensis |
81,114 |
|
59 |
6,7-dimethoxy-2-[2-(2-hydroxyphenyl)ethyl]chromone |
H |
OCH3 |
OCH3 |
H |
OH |
H |
H |
H |
A. sinensis |
53,92 |
|
60 |
6-methoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl] chromone |
H |
OCH3 |
H |
H |
H |
OCH3 |
OH |
H |
A. malaccensis A. sinensis A. crassna Aquilaria spp. |
85 53,92,88,105 106 104 |
|
61 |
6-hydroxy-7-methoxy-2-[2-(4-methoxyphenyl)ethyl] chromone |
H |
OH |
OCH3 |
H |
H |
H |
OCH3 |
H |
A. sinensis G. salicifolia A. filaria |
114 54 84 |
|
62 |
7-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl] chromone |
H |
H |
OH |
H |
H |
OCH3 |
OH |
H |
A. sinensis |
81 |
|
63 |
(R)-6,7-dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone |
H |
OCH3 |
OCH3 |
H |
H |
H |
H |
R-OH |
A. sinensis |
93,114 |
|
64 |
(S)-6,7-dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone |
H |
OCH3 |
OCH3 |
H |
H |
H |
H |
S-OH |
A. sinensis |
93,114 |
|
65 |
6,7-dimethoxy-2-[2-(4-hydroxyphenyl)ethyl]chromone [Qinanone G] |
H |
OCH3 |
OCH3 |
H |
H |
H |
OH |
H |
A. sinensis |
81,83,92,114 |
|
66 |
6,7-dimethoxy-2-[2-(4-methoxyphenyl)ethyl]chromone [AH8] |
H |
OCH3 |
OCH3 |
H |
H |
H |
OCH3 |
H |
Kalinantan A. sinensis A. malaccensis A. crassna |
100 29,53,56,62 34 102 |
|
67 |
7-chloro-8-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
H |
Cl |
OH |
H |
H |
OCH3 |
H |
A. sinensis |
53 |
|
68 |
8-chloro-6-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
H |
Cl |
H |
OH |
H |
H |
OCH3 |
H |
A. sinensis A. crassna |
93,110 63,106 |
|
69 |
5,8-dihydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl] chromone |
OH |
H |
H |
OH |
H |
OH |
OCH3 |
H |
G. salicifolia |
54 |
|
70 |
5,6-dihydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl] chromone |
OH |
OH |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis |
62 |
|
71 |
5,8-dimethoxy-2-[2-(3-acetoxyphenyl)ethyl]chromone |
OCH3 |
H |
H |
OCH3 |
H |
OCOCH3 |
H |
H |
A. agallocha |
97 |
|
72 |
6-methoxy-2-[2-(3,4,5-trihydroxyphenyl)ethyl]chromone |
H |
OCH3 |
H |
H |
OH |
OH |
OH |
H |
A. sinensis |
113 |
|
73 |
6,8-dihydrxoy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
H |
OH |
H |
OH |
H |
OH |
OCH3 |
H |
A. sinensis |
107,115 |
|
74 |
6,8-dihydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone |
H |
OH |
H |
OH |
H |
OCH3 |
OH |
H |
A. sinensis |
118 |
|
75 |
8-chloro-6-hydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
H |
OH |
H |
Cl |
H |
OH |
OCH3 |
H |
A. sinensis A. crassna |
92,93,114 106 |
|
76 |
2-[2-(4-glucosyloxy-3-methoxyphenyl)ethyl]chromone |
H |
H |
H |
H |
H |
OCH3 |
glu |
A. sinensis |
119 |
|
|
77 |
5-Methoxy-6-hydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
OCH3 |
OH |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis |
114 |
|
78 |
6,7-dimethoxy-2-[2-(3-methoxy-4-hydroxylphenyl)ethyl]chromone |
H |
OCH3 |
OCH3 |
H |
H |
OCH3 |
OH |
H |
A. sinensis Aquilaria spp. |
81,114,115 90 |
|
79 |
6-methoxy-7-hydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
H |
OCH3 |
OH |
H |
H |
OH |
OCH3 |
H |
A. sinensis G. salicifolia Aquilaria spp. |
115 111 75 |
|
80 |
5-hydroxy-6,7-dimethoxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
OH |
OCH3 |
OCH3 |
H |
H |
H |
OCH3 |
H |
A. sinensis |
91 |
|
81 |
6,7-dimethoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
H |
OCH3 |
OCH3 |
H |
H |
OH |
OCH3 |
H |
A. sinensis A. crassna |
81,114,115 102 |
|
82 |
6-hydroxy-7-methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
H |
OH |
OCH3 |
H |
H |
OH |
OCH3 |
H |
A. sinensis G. salicifolia Aquilaria spp. Aquilaria spp. |
81,107,114 111 90 75 |
|
83 |
7-hydroxyl-6-methoxyl-2-[2-(4-hydroxyl-3-methoxylphenyl)ethyl]chromone |
H |
OCH3 |
OH |
H |
H |
OCH3 |
OH |
H |
A. sinensis |
120 |
|
84 |
5-hydroxy-6-methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone |
OH |
OCH3 |
H |
H |
H |
OH |
OCH3 |
H |
A. sinensis A. crassna |
88 102 |
|
85 |
6-hydroxy-7-methoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone [Aquilarone G] |
H |
OH |
OCH3 |
H |
H |
OCH3 |
OH |
H |
A. sinensis Aquilaria spp. |
107 90 |
|
85a |
6,7-dihydroxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone |
- |
- |
- |
- |
- |
- |
- |
- |
Jinko |
79 |
|
86 |
6-methoxy-2-[2-(3,4,5-trihydroxyphenyl)ethyl]chromone |
H |
OCH3 |
H |
H |
OH |
OH |
OH |
H |
A. sinensis |
113 |
The commonly observed substituents in FPECs core structure are hydroxy and methoxy groups, and are substituted at C-6, followed by C-7, C-5 and C-8. The methoxy functional groups appear more frequently at C-7 than hydroxyl groups. However, it is interesting to note that five chlorinated FPECs (37, 53, 67, 68 and 75), are reported from the agarwood species (Figure 14, Table 10). It is also reported the only glycosylated FPEC (76) from A. sinensis. The only acetyl FPEC (71) reported from the agarwood of A. agallocha.
2.2.1b. 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (TPECs)
The isolated and structure identified highly oxidized TPECs (87–135) from agarwood species are presented as Figure 15, and Table 11. Further, chemical examination of whole-tree agarwood-inducing technique (Agar-Wit) from 8 years old A. sinensis, resulted in the isolation of TPEC compounds 135a, 135b, and 135c (Figure 14) [121].
Figure 15: General chemical structure of TPECs from agarwood species.
Table 11: 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (TPECs) reported from agarwood.
|
No. |
Name |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Source |
Ref. |
|
87 |
(6S,7S,8S)-6,7,8-trihydroxyl-2-(3-hydroxyl-4-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one. |
H |
α-OH |
α-OH |
α-OH |
H |
OH |
OCH3 |
H |
A. sinensis |
120 |
|
88 |
(6S,7S,8S)-6,7,8-trihydroxyl-2-(4-hydroxyl-3-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one |
H |
α-OH |
α-OH |
α-OH |
H |
OCH3 |
OH |
H |
A. sinensis |
120 |
|
89 |
6,7-dihydroxy-5,6,7,8-tetrahydro-2-(2-(4-methoxy phenyl)ethyl)chromone |
H |
α-OH |
α-OH |
H |
H |
H |
OCH3 |
H |
A. crassna |
122 |
|
90 |
6,7-dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone |
H |
α-OH |
α-OH |
H |
H |
H |
H |
H |
A. sinensis Aquilaria spp. |
95 90 |
|
91 |
(6S,7S,8R)-6,7-dihydroxy-8-chloro-5,6,7,8-tetrahydro-2-(2-(3-hydroxy-4-methoxyphenyl)ethyl)chromone |
H |
α-OH |
α-OH |
β-Cl |
H |
OH |
OCH3 |
H |
A. crassna |
122 |
|
92 |
(5S,6R,7R)-5,6,7-trihydroxy-2-(3-hydroxy-4-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one |
α-OH |
α-OH |
α-OH |
H |
H |
OH |
OCH3 |
H |
A. sinensis |
91,123 |
|
93 |
rel-(5R,6S,7R)-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one |
α-OH |
α-OH |
β-OH |
H |
H |
H |
OCH3 |
H |
A. malaccensis |
34 |
|
94 |
(6R,7S,8S)-6,7,8-trihydroxy-2-(4-hydroxyl-3-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one. |
β-OH |
β-OH |
β-OH |
H |
H |
OCH3 |
OH |
H |
A. sinensis |
91 |
|
95 |
rel-(5R,6S,7R)-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one |
α-OH |
α-OH |
β-OH |
H |
H |
H |
H |
H |
A. malaccensis |
34 |
|
96 |
(5S,6S,7R)-5,6,7-trihydroxy-2-[2-(hydroxylphenyl)ethyl]-5,6,7,8-tetrahydrochromone [AH9] |
α-OH |
β-OH |
α-OH |
H |
OH |
H |
H |
H |
Kalimantan |
100 |
|
97 |
(5S,6R,7S)-5,6,7-trihydroxy-2-(3-hydroxy-4-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one |
α-OH |
α-OH |
β-OH |
H |
H |
OH |
OCH3 |
H |
A. malaccensis |
34 |
|
98 |
Agarotetrol [AH1] |
α-OH |
β-OH |
β-OH |
α-OH |
H |
H |
H |
H |
A. agallocha Kalimantan Aquilaria spp. A. sinensis |
124 125 104 126, 127 |
|
99 |
Aquilarone B |
α-OH |
α-OH |
α-OH |
β-OH |
H |
H |
H |
H |
A. sinensis Aquilaria spp. |
107,127 90 |
|
100 |
Tetrahydrochromone B |
β-OCH3 |
α-OH |
α-OH |
β-OH |
H |
H |
H |
H |
A. sinensis |
127 |
|
101 |
5α,6β,7β-trihydroxy-8α-methoxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone [AH17] |
α-OH |
β-OH |
β-OH |
α-OCH3 |
H |
H |
H |
H |
Kalimantan A. sinensis |
128 127 |
|
102 |
(5R,6R,7S,8R)-2-(2-phenylethyl)-tetrahydroxy-5,6,7,8-tetrahydrochromone [AH16] |
β-OH |
β-OH |
α-OH |
β-OH |
H |
H |
H |
H |
Kalimantan A. sinensis |
129 126 |
|
103 |
Isoagarotetrol [AH2] |
α-OH |
β-OH |
α-OH |
β-OH |
H |
H |
H |
H |
Kalimantan A. sinensis Aquilaria spp. |
125 95 90 |
|
104 |
(5R,6S,7S,8R)-2-[2-(4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone |
β-OH |
α-OH |
α-OH |
β-OH |
H |
H |
OCH3 |
H |
Aquilaria spp. |
90 |
|
105 |
5α,6β,7α,8β-tetrahydroxy-2-[2-(4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone [AH2a] |
α-OH |
β-OH |
α-OH |
β-OH |
H |
H |
OCH3 |
H |
Kalimantan A. sinensis Aquilaria spp. |
130 37,87 90 |
|
106 |
(5S,6R,7S,8R,7'R)-7'-hydroxyisoagarotetrol |
α-OH |
β-OH |
α-OH |
β-OH |
H |
H |
H |
R-OH |
Kalimantan |
131 |
|
107 |
8-chloro-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydrochromone |
α-OH |
α-OH |
α-OH |
β-Cl |
H |
H |
H |
H |
A. sinensis Aquilaria spp. |
53,95 90 |
|
108 |
5α,6β,7α,8β-tetrahydroxy-2-[2-(2-hydroxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone [AH2b] |
α-OH |
β-OH |
α-OH |
β-OH |
OH |
H |
H |
H |
Kalimantan A. sinensis Aquilaria spp. |
130 56 90 |
|
109 |
5α,6β,7β,8α-tetrahydroxy-2-[2-(2-hydroxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (AH23) |
α-OH |
β-OH |
β-OH |
α-OH |
OH |
H |
H |
H |
Kalimantan |
128 |
|
110 |
5α,6β,7β,8α-tetrahydroxy-2-[2-(4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone[AH1A] [4'-methoxy-agarotetrol] |
α-OH |
β-OH |
β-OH |
α-OH |
H |
H |
OCH3 |
H |
Kalimantan A. sinensis Aquilaria spp. |
130 127,132 116 |
|
111 |
Aquilarone C |
α-OH |
α-OH |
α-OH |
β-OH |
H |
H |
OCH3 |
H |
A. sinensis Aquilaria spp. |
91,107 90 |
|
112 |
(5S,6S,7S,8S)-8-chloro-5,6,7-trihydroxy-2-(phenylethyl)-5,6,7,8-tetrahydrochromone |
α-OH |
α-OH |
α-OH |
α-Cl |
H |
H |
H |
H |
A. sinensis |
91 |
|
113 |
(5S,6R,7S,8R,7'S)-7'-hydroxyisoagarotetrol |
α-OH |
β-OH |
α-OH |
β-OH |
H |
H |
H |
S-OH |
Kalimantan |
131 |
|
114 |
Aquilarone F |
α-OH |
β-OH |
β-OH |
α-OH |
H |
H |
OH |
H |
A. sinensis Aquilaria spp. |
107 90 |
|
115 |
(5R,6S,7S,8R)-2-[2-(4-hydroxy-3- methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone |
β-OH |
α-OH |
α-OH |
β-OH |
H |
OCH3 |
OH |
H |
Aquilaria spp. |
90 |
|
116 |
Tetrahydrochromone G |
β-OCH3 |
β-OH |
β-OH |
α-OH |
H |
H |
OCH3 |
H |
A. sinensis |
127 |
|
117 |
Aquilarone E |
α-OH |
β-OH |
β-OH |
α-OH |
H |
OH |
OCH3 |
H |
A. sinensis |
107, 127 |
|
118 |
(5R,6S,7S,8R)-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone |
β-OH |
α-OH |
α-OH |
β-OH |
H |
OH |
OCH3 |
H |
Aquilaria spp. |
90 |
|
119 |
Tetrahydrochromone F |
α-OCH3 |
α-OH |
α-OH |
β-OH |
H |
H |
OCH3 |
H |
A. sinensis A. crassna |
127 102 |
|
120 |
(5R,6S,7S,8R)-5,6,7-trihydroxy-8-methoxy-5,6,7,8-tetrahydro-2-(2-(4-methoxyphenyl)ethyl)chromone |
β-OH |
α-OH |
α-OH |
β-OCH3 |
H |
H |
OCH3 |
H |
A. crassna |
122 |
|
121 |
Tetrahydrochromone E |
α-OH |
β-OH |
β-OH |
α-OCH3 |
H |
H |
OCH3 |
H |
A. sinensis |
127 |
|
122 |
5,6,7,8-tetrahydroxy-2-(3-hydroxy-4-methoxy phenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one |
α-OH |
β-OH |
β-OH |
α-OH |
H |
OH |
OCH3 |
H |
A. sinensis |
133 |
|
123 |
Aquilarone D |
α-OH |
β-OH |
α-OH |
β-OH |
H |
OH |
OCH3 |
H |
A. sinensis Aquilaria spp. |
56,107 90 |
|
124 |
Aquilarone A |
α-OH |
α-OH |
α-OH |
β-OH |
H |
OH |
OCH3 |
H |
A. sinensis Aquilaria spp. |
107,127 90 |
|
125 |
Tetrahydrochromone A |
α-OCH3 |
β-OH |
β-OH |
α-OH |
H |
H |
OCH3 |
H |
A. sinensis |
127 |
|
126 |
(5R,6R,7R,8R)-8-chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone |
β-OH |
β-OH |
β-OH |
β-Cl |
H |
H |
OCH3 |
H |
A. sinensis |
91 |
|
127 |
rel-(5R,6S,7S,8R)-8-chloro--5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one |
α-OH |
β-OH |
β-OH |
α-Cl |
H |
H |
OCH3 |
H |
A. malaccensis A. sinensis |
34 127 |
|
128 |
(5R,6R,7R,8S)-8-chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone |
β-OH |
β-OH |
β-OH |
α-Cl |
H |
H |
OCH3 |
H |
A. sinensis Aquilaria sp |
91 75 |
|
129 |
Tetrahydrochromone I |
α-OCH3 |
α-OH |
α-OH |
β-Cl |
H |
H |
OCH3 |
H |
A. sinensis |
127 |
|
130 |
Tetrahydrochromone D |
α-OCH3 |
β-OH |
β-OH |
α-Cl |
H |
H |
OCH3 |
H |
A. sinensis |
127 |
|
131 |
Tetrahydrochromone C |
α-OCH3 |
β-OH |
β-OH |
α-OH |
H |
OH |
OCH3 |
A. sinensis |
127 |
|
|
132 |
Tetrahydrochromone H |
α-OCH3 |
α-OH |
α-OH |
β-OH |
H |
OH |
OCH3 |
H |
A. sinensis |
127 |
|
133 |
Tetrahydrochromone J |
α-OCH3 |
α-OH |
α-OH |
β-Cl |
H |
OH |
OCH3 |
H |
A. sinensis |
127 |
|
134 |
8-chloro-5,6,7-trihydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromon-one |
α-OH |
α-OH |
α-OH |
β-Cl |
H |
OH |
OCH3 |
H |
A. sinensis |
134 |
|
135 |
rel-(5R,6S,7S,8R)-8-chloro-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]- 4H-1-benzopyran-4-one |
α-OH |
β-OH |
β-OH |
α-Cl |
H |
OH |
OCH3 |
H |
A. malaccensis A. sinensis |
34 37 |
|
135a |
(5S,6R,7S,8S)-8-chloro-5,6,7-trihydroxy-2-[2-(4′-methoxyphenylethyl)]-5,6,7,8-tetrahydrochromone |
α-OH |
β-OH |
α-OH |
α-Cl |
H |
H |
OCH3 |
H |
A. sinensis |
121 |
|
135b |
(5S,6R,7S,8S)-8-chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8- tetrahydrochromone |
α-OH |
β-OH |
α-OH |
α-Cl |
H |
H |
H |
H |
A. sinensis |
121 |
|
135c |
(5S,6R,7R,8S)-8-chloro-5-ethoxy-6,7-dihydroxy-2-[2-(3′-hydroxy-4′-methoxy-phenylethyl)-5,6,7,8-tetrahydrochromone |
α-OCH2CH3 |
β-OH |
β-OH |
α-Cl |
H |
OH |
OCH3 |
H |
A. sinensis |
121 |
Among the reported TPECs, the compound, agarotetrol (98) is a common metabolite found in different samples of agarwood. The Chinese Pharmacopeia (2015 edition), identified agarotetrol (98) as a marker compound of agarwood, and standardized the contents needs to be higher than 0.10%. Most of the reported TPECs are oxidized at C-5, C-6, C-7 and C-8 positions, either with hydroxy or methoxy functional groups. It is observed that the reported TPECs at C-6 and C-7 are usually substituted with hydroxyl groups, while the methoxy group at C-5 or C-8. Some of the reported TPECs contains a chlorine substituent at the C-8 position. It is interesting to note that the TPECs 106 and 113 are ethoxy derivatives.
2.2.1c. Mono-epoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (EPECs)
Various epoxy-substituted PECs are reported from agarwood species (Figure 16, Table 12). The epoxy group is usually located either at C-5 and C-6, or at C-7 and C-8. However, the EPEC 141 carries an epoxy group located at C-6 and C-7 [135]. Similar to the structures of TPECs, the C-5, C-6, C-7 and C-8 positions of EPECs are oxidized and carry hydroxy or methoxy or epoxy groups, while the methoxy groups usually located at C-5 or C-8. In this connection, the EPECs 136‒138 are reported from A. malaccensis [34], whereas 136‒144, and 148‒150 are reported from the agarwood of A. sinensis (Table 12). The EPECs 136 and 144 are obtained from the agarwood of A. crassna [108].
2.2.1d. Diepoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones (DPECs)
Various DPECs compounds (145–151), are reported from the species of agarwood (Figure 16, Table 12). The DPECs (145–147) are obtained from the agarwood of A. crassna, A. malaccensis and A. sinensis (Table 12).
Figure 16: Chemical structures of EPECs and DPECs of agarwood.
Table. 12: EPECs and DPECs from agarwood species.
|
No. |
Name |
Source |
Ref. |
|
136 |
rel-(1aR,2R,3R,7bS)-1a,2,3,7b-tetrahydro-2,3-dihydroxy-5-[2-(4-methoxyphenyl)ethyl]-7H-oxireno[f] [1]benzopyran-7-one |
A. malaccensis A. sinensis A. crassna |
34 91 108 |
|
137 |
rel-(1aR,2R,3R,7bS)-1a,2,3,7b-tetrahydro-2,3-dihydroxy-5-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-7H-oxireno[f][1]benzopyran-7-one |
A. malaccensis A. sinensis |
34 88 |
|
138 |
rel-(1aR,2R,3R,7bS)-1a,2,3,7b-Tetrahydro-2,3-dihydroxy-5-(2-phenylethyl)-7H-oxireno[f] [1]benzopyran-7-one |
A. malaccensis A. sinensis |
34,82 53,88 |
|
139 |
5,6-epoxy-7β-hydroxy-8β-methoxy-2-(2-phenylethyl)chromone |
A. sinensis |
88 |
|
140 |
5α,6α-Epoxy-7β,8α,3′-trihydroxy-4′-methoxy-2-(2-phenylethyl)chromone |
A. sinensis |
113 |
|
141 |
(5S,6R,7S,8S)-2-[2-(4′-methoxyphenyl)ethyl]-6,7-epoxy-5,8-dihydroxy-5,6,7,8-tetrahydrochromone |
A. sinensis |
135 |
|
142 |
Tetrahydrochromone M |
A. sinensis |
127 |
|
143 |
Tetrahydrochromone L |
A. sinensis |
127 |
|
144 |
(5R,6S,7S,8S)-2-[2-(4′-methoxyphenyl)ethyl]-7,8-epoxy-5-methoxy-6-hydroxy-5,6,7,8-tetrahydrochromone |
A. sinensis A. crassna |
135 108 |
|
145 |
Oxidoagarochromone B |
A. crassna A. malaccensis A. sinensis |
136 34 53,88 |
|
146 |
Oxidoagarochromone A |
A. crassna A. malaccensis A. sinensis |
136 34 53,88 |
|
147 |
Oxidoagarochromone C |
A. crassna A. malaccensis A. sinensis |
136 34 127 |
|
148 |
(5S,6S,7S,8S)-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]-7,8-epoxy-5-methoxy-6-hydroxy-5,6,7,8- tetrahydrochromone |
A. sinensis |
135 |
|
149 |
(5S,6S,7S,8S)-2-[2-(4′-methoxyphenyl)ethyl]-7,8-epoxy-5-methoxy-6-hydroxy-5,6,7,8-tetrahydrochromone |
A. sinensis |
135 |
|
150 |
(5S,6S,7S,8S)-2-[2-(4′-methoxyphenyl)ethyl]-7,8-epoxy-5,6-dihydroxy-5,6,7,8-tetrahydrochromone |
A. sinensis |
135 |
|
151 |
Tetrahydrochromone K |
A. sinensis |
127 |
The C-4′ position of the phenylethyl moiety in both EPECs and DPECs are without substitution, or substituted with a methoxy group (Figure 16). Alternatively, both EPECs and DPECs are substituted at C-3′ with a hydroxyl and/or C-4′ with a methoxyl group (Figure 16).
2.2.1e. Other PECs
From the agarwood species of A. crassna, A. filaria, A. sinensis, and G. salicifolia, seven 2-(2-phenylethenyl)chromones (PEECs) are reported (Figure 17). The compounds PEECs are possess a styryl moiety instead of a phenylethyl moiety at C-2 of chromones. The chemical structures and names are presented as in Figure 17 and Table 13.
Figure 17: Chemical structures of 2-(2-phenylethenyl)chromones (PEECs) from agarwood.
Table 13: 2-(2-phenylethenyl)chromones (PEECs) from agarwood.
|
No. |
Name |
Source |
Ref. |
|
152 |
(E)-2-[2-(3-methoxy-4-hydroxyphenyl)ethenyl]chromone |
A. crassna |
109 |
|
153 |
(E)-6-methoxy-2-[2-(4-hydroxyphenyl)ethenyl]chromone |
A. crassna |
109 |
|
154 |
5-hydroxy-2-[2-(4-methoxyphenyl)ethenyl]chromone |
G. salicifolia A. filaria |
54 68 |
|
155 |
(E)-6-methoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethenyl] chromone |
A. crassna |
109 |
|
156 |
6-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethenyl]chromone |
A. sinensis Aquilaria spp. |
37,115 116 |
|
157 |
5-hydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethenyl]chromone |
G. salicifolia |
137 |
|
158 |
6,7-dimethoxy-2-[2-(4-hydroxyphenyl)ethenyl]-4H-chromen-4-one |
A. sinensis |
92 |
2.2.2. Dimeric 2-(2-phenylethyl)chromones (DIPECs)
The agarwood species of A. sinensis and A. crassna are rich source for the compounds DIPECs (Figure 18, Table 14). The DIPECs are isolated and purified by silica gel column chromatography and semi-preparetive HPLC and so on. The chemical structures of DIPECs are identified by spectroscopic data. The single C–C bond linked DIPECs (159–162), which are composed of two FPECs (unit A and unit B) through a C5–C5′ linkage, are reported from the agarwood “Jinko” from Kalimantan (Figure 18, Table 14). Further, chemical examination of whole-tree agarwood-inducing technique (Agar-Wit) from 8 years old A. sinensis, resulted in the isolation of a single C–C bond linked DIPEC, aqulisinone A (162a, Figure 18) [121]. The reported remaining DIPECs are linked with a C–O–C bond (Table 14). Most of these C–O–C bond linked DIPECs are composed of a TPEC unit (unit A) and a FPEC unit (unit B) (Figure 18, Table 14). In the DIPECs 167–187, the linkage position of unit A is situated at C-8, while the linkage position of unit B is usually at C-7′ or at C-6′, except for dimer 176 (Figure 18). The DIPECs 195 and 196 are composed of two EPEC units, which are connected through a C5–O–C6′ linkage (Figure 18). It is observed that the DIPECs unit A usually linked at C5 or C8. The reason might be that the conjugated C5 and C8 of 4H-pyran-4-one yielded the stable intermediate carbocations as compared with C6 and C7. The DIPECs AH10‒AH15, and AH21 (164‒166, 168, and 169) are reported from the agarwood “Jinko” from Kalimantan (Table 15). Further, AH10 (166) and AH14 (165) are also reported from the withered wood of A. sinensis grown in Taiwan [95]. A recent study reported the new DIPECs 198a‒198d from the MeOH extract of agarwood Jinko [79]. The DIPEC compounds, aquilasinenones L and M (198e and 198f) are reported from the artificial agarwood originating from A. sinensis [138].
Figure 18: Chemical structures of dimeric 2-(2-phenylethyl)chromones from agarwood.vvv
Table 14: Dimeric 2-(2-phenylethyl)chromones with C–C bond of agarwood.
|
No. |
Name |
Source |
Ref. |
|
159 |
Aquisinenone O |
A. sinensis |
139 |
|
160 |
2,2′-di-(2-phenylethyl)-8,6′-dihydroxy-5,5′-bichromone [AH11] |
Kalimantan A. sinensis |
140 139 |
|
161 |
7,4′-dimethoxyaquisinenone O |
A. sinensis |
139 |
|
162 |
Crassin A |
A. crassna A. sinensis |
141 139 |
|
162a |
Aqulisinone A |
A. sinensis |
121 |
Table 15: Dimeric 2-(2-phenylethyl)chromones with C–O–C bond of agarwood.
|
No. |
Name |
Source |
Ref. |
|
163 |
Aquilasinenone K |
A. sinensis |
142 |
|
164 |
(5S,6S,7R,8S)-2-(2-phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)-7-hydroxy-chromonyl-6-oxy]chromone [AH15] |
Kalimantan |
143 |
|
165 |
(5S,6R,7S,8S)-2-(2-phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethy)chromonyl-6-oxy]chromone [AH14] |
Kalimantan A. sinensis |
144 39,95 |
|
166 |
(5S,6S,7R,8S)-2-(2-phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethy)chromonyl-6-oxy]chromone [AH10] |
Kalimantan A. sinensis |
140 95 |
|
167 |
(5R,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone |
A. sinensis |
145 |
|
168 |
(5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethy)chromonyl-6-oxy]chromone [AH13] |
Kalimantan |
144 |
|
169 |
(5R,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethy)-7-methoxychromonyl-6-oxy]chromone [AH12] |
Kalimantan |
144 |
|
170 |
Aquisinenone N |
A. sinensis |
139 |
|
171 |
(5S,6R,7S,8R)-2-[2-(4-methoxyphenyl)ethyl]-5,6,7-trihydroxy5,6,7,8-tetrahydro-8-{2-[2-(4‴-methoxyphenyl)ethyl]chromonyl-6-oxy}chromone |
A. sinensis |
145 |
|
172 |
(5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone |
A. sinensis |
145 |
|
173 |
Aquilasinenone J |
A. sinensis |
142 |
|
174 |
Aquisinenone M |
A. sinensis |
139 |
|
175 |
Crassin D |
A. sinensis |
139 |
|
176 |
Crassin B |
A. sinensis |
141 |
|
177 |
Aquilasinenone C |
A. sinensis |
142 |
|
178 |
Aquilasinenone B |
A. sinensis |
142 |
|
179 |
Aquilasinenone E |
A. sinensis |
142 |
|
180 |
Aquilasinenone D |
A. sinensis |
142 |
|
181 |
Aquilasinenone A |
A. sinensis |
142 |
|
182 |
Crassin C |
A. crassna |
141 |
|
183 |
Aquilasinenone H |
A. sinensis |
142 |
|
184 |
Aquilasinenone G |
A. sinensis |
142 |
|
185 |
Aquilasinenone I |
A. sinensis |
142 |
|
186 |
(5S,6R,7S,8R)-2-[2-(4-methoxyphenyl)ethyl]-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-{6-methoxy-2-[2-(3‴-methoxy-4‴-hydroxyphenyl)ethyl]chromonyl-7-oxy}chromone |
A. sinensis |
145 |
|
187 |
Aquilasinenone F |
A. sinensis |
142 |
|
188 |
Aquisinenone H |
A. sinensis |
139 |
|
189 |
4′-methoxyaquisinenone |
A. sinensis |
139 |
|
190 |
4′,7″,4‴-trimethoxyaquisinenone I |
A. sinensis |
139 |
|
191 |
Aquisinenone I |
A. sinensis |
139 |
|
192 |
7″-methoxyaquisinenone I |
A. sinensis |
139 |
|
193 |
4′,7″-dimethoxyaquisinenone I |
A. sinensis |
139 |
|
194 |
Aquisinenone L |
A. sinensis |
139 |
|
195 |
4′,4‴-dimethoxyaquisinenone K |
A. sinensis |
139 |
|
196 |
Aquisinenone K |
A. sinensis |
139 |
|
197 |
Aquisinenone J |
A. sinensis |
139 |
|
198 |
4′-methoxyaquisinenone J |
A. sinensis |
139 |
|
198a |
(5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[6′-hydroxy-2-(2-phenylethyl)chromonyl-7′-oxy]chromone [diaquilariachrome A] |
Jinko |
79 |
|
198b |
(5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[6′-hydroxy-2-(2-phenylethyl)chromonyl-8′-oxy]chromone [diaquilariachrome B] |
Jinko |
79 |
|
198c |
(5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-(3-phenylpropionyloxy)chromone |
Jinko |
79 |
|
198d |
(5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-{6′-hydroxy-2-[2-(4″′-methoxyphenyl)ethyl]chromonyl-7′-oxy}chromone [diaquilariachrome C] |
Jinko |
79 |
|
198e |
Aquilasinenones L |
A. sinensis |
138 |
|
198f |
Aquilasinenones M |
A. sinensis |
138 |
Additionally, double linked 2-(2-phenethyl)chromone dimers (DLPECs) also reported from the species of agarwood. The reported compounds chemical structures (Figure 19) are presented in Table 16. These compounds are composed of a TPEC unit (unit A) and an FPEC unit (unit B) (Figure 19). In most of the DLPECs, the linkage position of unit A is usually at C5 and C7, while the same in unit B is C6′ and C7′, might be duce to the 6,7-dihydroxy-FPECs provides two adjacent hydroxyl groups to form the two C–O–C bonds (Figure 19). In the TPEC unit, the C‒O‒C bond linked at C7, while the same for the C‒C bond is at C5. On the other hand, in the FPEC unit, the C–C bond linked at C5′, C7′ or C8′ of the chromone moiety (205–215), or at C2′′′, C3′′′ or C4′′′ of the phenylethyl moiety (216–224) (Figure 19, Table 16). Among these DLPECs, six compounds (199–204) are linked through two C–O–C bonds to form a seven or six-membered oxygen-carrying heterocyclic ring (Figure 19, Table 16). Six new DLPECs (204a–204f) are reported from the EtOAc extract of artificial agarwood originating from A. sinensis [146]. On the other hand, the DLPECs 205–224 contains an unusual 3,4-dihydro-2H-pyran ring connected to two PEC monomeric moieties through a C–O–C bond and a C–C bond (Figure 19, Table 17). Three new C–O–C bond DLPECs, crassin I‒ K (224a‒224c) (Figure 19, Table 17), reported from the artificial holing agarwood originating from A. sinensis [147].
Table 16: Double linked 2-(2-phenylethyl)chromones with double C–O–C bonds.
|
No. |
Name |
Source |
Ref. |
|
199 |
Crassin E |
A. crassna |
148 |
|
200 |
Crassin F |
A. crassna |
148 |
|
201 |
Crassin G |
A. crassna |
148 |
|
202 |
AH21 |
Kalimantan |
149 |
|
203 |
(+)-4′-Methoxyaquisinenone G |
A. sinensis |
150 |
|
204 |
(−)-Aquisinenone G |
A. sinensis |
150 |
|
204a |
(−)-3′′′-hydroxy-4′′′-methoxy-aquisinenone G |
A. sinensis |
146 |
|
204b |
(+)-3′′′-hydroxy-4′′,4′′′-dimethoxy-aquisinenone G |
A. sinensis |
146 |
|
204c |
(+)-3′′-hydroxy-4′′,4′′′-dimethoxy-aquisinenone G |
A. sinensis |
146 |
|
204d |
(+)-4′′′-hydroxy-4′′,3′′′-dimethoxy-aquisinenone G |
A. sinensis |
146 |
|
204e |
3′′′-hydroxy-4′′-demethoxy-crassin G |
A. sinensis |
146 |
|
204f |
3′′′-hydroxy-crassin G |
A. sinensis |
146 |
Figure 19: Chemical structures of agarwood double linked 2-(2-phenylethyl) chromones.
Table 17: Double linked 2-(2-phenylethyl)chromones with C–O–C and C–C bonds.
|
No. |
Name |
Source |
Ref. |
|
205 |
(−)-Aquisinenone C |
A. sinensis |
150 |
|
206 |
(+)-Aquisinenone C |
A. sinensis |
150 |
|
207 |
(+)-6″-hydroxy-4′,4‴-dimethoxyaquisinenone B |
A. sinensis |
150 |
|
208 |
(+)-Aquisinenone B |
A. sinensis |
150 |
|
209 |
(−)-6″-hydroxyaquisinenone B |
A. sinensis |
150 |
|
210 |
(−)-Aquisinenone B |
A. sinensis |
150 |
|
211 |
Aquisinenone P |
A. crassna |
151 |
|
212 |
Aquisinenone Q |
A. crassna |
151 |
|
213 |
(+)-Aquisinenone A |
A. sinensis A. crassna |
150 151 |
|
214 |
(−)-Aquisinenone A |
A. sinensis A. crassna |
150 151 |
|
215 |
(−)-4′-methoxyaquisinenone A |
A. sinensis |
150 |
|
216 |
(−)-Aquisinenone D |
A. sinensis A. crassna |
150 151 |
|
217 |
(−)-4′-demethoxyaquisinenone D |
A. sinensis A. crassna |
150 106 |
|
218 |
(+)-4′-demethoxyaquisinenone D |
A. sinensis A. crassna |
150 106 |
|
219 |
3′-hydroxyaquisinenone D |
A. crassna |
122 |
|
220 |
Aquisinenone R |
A. crassna |
151 |
|
221 |
(+)-Aquisinenone D |
A. crassna |
151 |
|
222 |
Crassin H |
A. crassna |
148 |
|
223 |
(−)-Aquisinenone F |
A. sinensis |
150 |
|
224 |
(+)-Aquisinenone E |
A. sinensis |
150 |
|
224a |
Crassin I |
A. sinensis |
147 |
|
224b |
Crassin J |
A. sinensis |
147 |
|
224c |
Crassin K |
A. sinensis |
147 |
2.2.3. Sesquiterpenoid-4H-chromones (STCc) and benzylacetone-4H-chromones (BACs)
It is reported that the rare sesquiterpenoid-4H-chromone derivatives (STCs, 225–234) are reported from the species of agarwood (Figure 20, Table 18). These STCs are composed of a PEC (unit A) and a sesquiterpene moiety (unit B) linked together by an ester bond or an ether bond (Figure 20, Table 18). The A. crassna agarwood collected in Laos contains STCs of 225–230, which are composed by the coupling of a sesquiterpene moiety (unit B) at the C-8 position of the TPEC (unit A) by an ester bond [152]. The only qinanmer STC 231 is a sesquiterpenoid-4H-chromone reported from the Chinese agarwood “Lv Qi-Nan” of A. sinensis.126 The Cambodian variety of A. crassna agarwood resulted the STCs of 232–234, which are formed by the sesquiterpene moiety connected to the EPEC through an ether bond [151]. Furthermore, the benzylacetone-4H-chromone derivatives 235 and 236 are reported from the agarwood of G. salicifolia [137]. These STCs are consisting an unusual 3,4-dihydro-2H-pyran ring, which is formed by a C‒O‒C bond and a C‒C bond at C-7 and C-5, respectively (Figure 20).
Figure 20: Sesquiterpenoid-4H-chromones and benzylacetone-4H-chromones of agarwood.
Table 18: Sesquiterpenoid-4H-chromones from agarwood
|
No. |
Name |
Source |
Ref. |
|
225 |
Aquilacrassnin D |
A. crassna |
152 |
|
226 |
Aquilacrassnin C |
A. crassna |
152 |
|
227 |
Aquilacrassnin B |
A. crassna |
152 |
|
228 |
Aquilacrassnin A |
A. crassna |
152 |
|
229 |
Aquilacrassnin F |
A. crassna |
152 |
|
230 |
Aquilacrassnin E |
A. crassna |
152 |
|
231 |
Qinanmer |
A. sinensis |
126 |
|
232 |
Xcrassin C |
A. crassna |
151 |
|
233 |
Xcrassin A |
A. crassna |
151 |
|
234 |
Xcrassin B |
A. crassna |
151 |
2.2.4. Trimers
The trimeric 2-(2-phenethyl)chromone compounds, AH19b (237), AH20 (238), AH18 (239), and AH19a (240) are reported from the agarwood “Jinko” from Kalimantan (Figure 21 and Table 19). The trimers 237, 239 and 240 are composed of two TPEC units (unit A and unit B) connected with a 6,7-dihydroxy-2-(2-phenethyl)chromone moiety (unit C) through a 5C–O–6C bond and a 5C–O–7C bond, respectively. The trimer 238 is composed of two TPEC units (A and B) with a 5,8-dihydroxy-2-(2-phenylethyl)chromone (unit C) through a 5C–O–8C bond and a 6C–O–5C bond, respectively (Figure 21).
Table 19: Tri-2-(2-phenylethyl)chromones of agarwood.
|
No. |
Name |
Source |
Ref. |
|
237 |
AH19b |
Kalimantan |
153 |
|
238 |
AH20 |
Kalimantan |
128 |
|
239 |
(5S,6S,7R,8S)-2-(2-phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)chromonyl-6,7-dioxy]chromone [AH18] |
Kalimantan |
143 |
|
240 |
AH19a |
Kalimantan |
153 |
Figure 21: Chemical structures of agarwood tri-2-(2-phenylethyl)chromones.
2.2.5. Phenolics and miscellaneous compounds
The volatile oils of agarwood contains various phenylbutanoids including, anisylacetone (P5), zingerone (P6), and benzylacetone (P7) (Figure 22) [29,48,154,155]. These phenylbutanoids generally are substituted with 3′, or 4′-OCH3, or 4′-OH, or withour any substitution (Figure 22). In this connection, it is interesting to note that the volatile aromatic compounds are reported from the smoke of agarwood, which might be the degradation products of chromones and lignins. Further, the phenylpropanoid such as 4′-methoxycinnamic acid (P1), 4′-methoxy-phenylpropionic acid (P4), anisic acid (P8), 3-hydroxy-4-methoxy phenylpropionic acid methyl ester (P9), and cinnamaldehyde (P15) are also reported from agarwood essential oils [24,48,56,65,155,156]. Additionally, the phenolic compounds including syringin (P10), and P11 ‒ P14 are also reported from the species of agarwood (Figure 22).
Figure 22: Phenolic compounds of agarwood.
On the other hand, the oxygen-containing triterpenoids, 3β-olean-12-ene-3,23-diol (M1), 3-oxo-22-hydroxyhopane (M2) and hederagenin (M3) (Fig. 23) are reported from the agarwood of A. sinensis[47,53,61,117,155].
Figure 23: Chemical structures of agarwood triterpenoid compounds.
2.3. Chemical constituents of Taiwan agarwood Excoecaria formosana
Excoecaria formosana (Syn: Excoecaria crenulata var. formosana Hayata), is a species of flowering plant in the family Euphorbiaceae. It is a shrub and mainly distributed in Tonkin, Indo-China, the southern part of Taiwan in thickets and forests along the seashores. The resin of Excoecaria is used as a substitute for agarwood incense. Chemical investigations on this plant resulted in the isolation of structurally diverse compounds. The halimane-type diterpenoids, formosins A–C (N1–N3), and and clerodane-type diterpenoids formosins D–F (N4–N6), are isolated from the 95% ethanolic extract of the twigs of E. formosana [157]. The whole plant of E. formosana led to the isolation of various metabolites; one apocarotenoid, seven benzenoids, cerebrosides, three coumarins, six coumarinolignans, three diterpenes, two flavonoids, six steroids, and eight galloyl glucosides (N7‒N50, Figure 24, Table 20) [158].
Figure 24: Chemical structures of Excoecaria formosana constituents.
Table 20: Chemical constituents of Excoecaria formosana.
|
No. |
Name |
Source |
Ref. |
|
N1 |
Formosin A |
E. formosana |
157 |
|
N2 |
Formosin B |
E. formosana |
157 |
|
N3 |
Formosin C |
E. formosana |
157 |
|
N4 |
Formosin D |
E. formosana |
157 |
|
N5 |
Formosin E |
E. formosana |
157 |
|
N6 |
Formosin F |
E. formosana |
157 |
|
N7 |
7α-hydroperoxysitosterol-3-O- β-D-(6-O-palmitoyl)glucopyranoside |
E. formosana |
158 |
|
N8 |
Excoecoumarin A |
E. formosana |
158 |
|
N9 |
Excoecoumarin B |
E. formosana |
158 |
|
N10 |
Excoeterpenol A |
E. formosana |
158 |
|
N11 |
Deglucosyl lauroside B |
E. formosana |
158 |
|
N12 |
Gallic acid |
E. formosana |
158 |
|
N13 |
Methyl gallate |
E. formosana |
158 |
|
N14 |
4-methoxybenzoic acid |
E. formosana |
158 |
|
N15 |
3-hydroxy-1-(3,5-dimethoxy-4-hydroxyphenyl)propan-1-one |
E. formosana |
158 |
|
N16 |
3-hydroxy-1-(4-hydroxy-3-ethoxyphenyl)propan-1-one |
E. formosana |
158 |
|
N17 |
2,3-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)propan-1-one |
E. formosana |
158 |
|
N18 |
(2S,3R)-4E-dehydrochebulic acid trimethyl ester |
E. formosana |
158 |
|
N19 |
Gynuramide I |
E. formosana |
158 |
|
N20 |
Gynuramide II |
E. formosana |
158 |
|
N21 |
Gynuramide III |
E. formosana |
158 |
|
N22 |
Gynuramide IV |
E. formosana |
158 |
|
N23 |
Scopoletin |
E. formosana |
158 |
|
N24 |
Fraxetin |
E. formosana |
158 |
|
N25 |
6-hydroxy-5,7-dimethoxycoumarin |
E. formosana |
158 |
|
N26 |
Cleomiscosins A |
E. formosana |
158 |
|
N27 |
Cleomiscosins B |
E. formosana |
158 |
|
N28 |
Cleomiscosins C |
E. formosana |
158 |
|
N29 |
Cleomiscosins D |
E. formosana |
158 |
|
N30 |
Malloapelin A |
E. formosana |
158 |
|
N31 |
Malloapelin B |
E. formosana |
158 |
|
N32 |
ent-11α-hydroxy-3-oxo-13-epi-manoyloxide |
E. formosana |
158 |
|
N33 |
Excoecafolin D |
E. formosana |
158 |
|
N34 |
Agallochin I |
E. formosana |
158 |
|
N35 |
(+)-catechin |
E. formosana |
158 |
|
N36 |
Kaempferol-3-O- β-D-glucoside |
E. formosana |
158 |
|
N37 |
6′-(stigmast-5-en-7-one-3-O-β -glucopyransidyl)hexadecanoate |
E. formosana |
158 |
|
N38 |
(6′-O-palmitoyl) sitosterol-3-O- β-D-glucoside |
E. formosana |
158 |
|
N39 |
β-sitosterol |
E. formosana |
158 |
|
N40 |
Stigmasterol |
E. formosana |
158 |
|
N41 |
3-O-β -D-glucopyranosyl β -sitosterol |
E. formosana |
158 |
|
N42 |
3-O-β -D-glucopyranosyl stigmasterol |
E. formosana |
158 |
|
N43 |
Isopropyl O-β -(6′-O-galloyl)glucopyranoside |
E. formosana |
158 |
|
N44 |
4-hydroxy-3-methoxyphenol 1-O-β -D-(2′,6′-di-O-galloyl)glucoside |
E. formosana |
158 |
|
N45 |
3-methoxy-4-hydroxyphenyl 1-O-β -D-(6′-O-galloyl)glucopyranoside |
E. formosana |
158 |
|
N46 |
1,2,3,4,6-penta-O-galloyl-β -D-glucose |
E. formosana |
158 |
|
N47 |
Corilagin |
E. formosana |
158 |
|
N48 |
1,4,6-tri-O-galloyl- β-D-glucose |
E. formosana |
158 |
|
N49 |
1,3,6-tri-O-galloyl-β -D-glucose |
E. formosana |
158 |
|
N50 |
Gallic acid 4-O-β -D-(6′-O-galloyl)-glucose |
E. formosana |
158 |
3. PHARMACOLOGICAL ACTIVITIES OF AGARWOOD
The agarwood-isolated compounds/extracts showed various pharmacological activities including, anti-inflammatory, anti-allergic, anti-diabetic, anti-cancer, anti-oxidant, anti-ischemic, anti-microbial, and effects on the central nervous system [3,6,12,25]. The details are described below.
3.1. Anti-inflammatory activity of agarwood compounds/extracts
Inflammation is a vital biological phenomenon that occurs in response to internal and external injurious stimuli to mitigate foreign triggers, initiate damaged tissue repair and restore the normal body homeostasis [159]. Although the healthy body requires limited inflammation, however, excessive inflammation can cause chronic and degenerative diseases such as diabetes, atherosclerosis, rheumatoid arthritis, cancer and cardiovascular diseases [159]. Nitric oxide (NO) is a pro-inflammatory mediator that plays a vital role in the process of inflammation [160]. Further, nuclear factor (NF)-κB and tumor necrosis factor (TNF)-α are the key cytokines involved in promoting and triggering the inflammatory process [160]. Therefore, inhibitors of NO, TNF-α, and NF-κB release may be considered as therapeutic targets for various inflammatory related ailments [160]. Agarwood compounds/extracts are examined for their anti-inflammatory activity through inhibition of NO, TNF-α, and NF-κB release in activated macrophages and neutrophils. Agarwood essential oil has an anti-inflammatory function, significantly reducing the skin thickness, ear weight, oxidative stress, and pro-inflammatory cytokines production in the 12-O-tetradecanoylphorobol-13 acetate (TPA)-induced mouse ear inflammation model [161]. The results of NO inhibitory production are summarized in Table 21.
The sesquiterpenoids, E17, E26 and E28 (Figure 7), are reported as inhibitors of NF-kB activation in the activated RAW264.7/Luc-P1 cell line, however, these compounds had not affect the NO release in LPS activated RAW264.7 macrophages (Table 21). Further, the sesquiterpenoids D4 (Figure 6), and E28 (Figure 7), at a concentration of 50 µM, suppress the superoxide anion generation in fMLP-activated human neutrophils [53]. On the other hand, among the reported PECs, the compounds 11, 16 and 48 (Figure 14), inhibited the LPS-induced NO production in RAW264.7 macrophages and NF-kB activation in the RAW264.7/Luc-P1 cell line [62]. The authors reported that the presence of a 6-methoxy moiety increased the activity, while the C4′ hydroxylation decreased. The PECs 9, 37, 47 and 75 reduced the release of TNF-α in LPS-activated RAW264.7 cells.93 Further, the PECs 6, 26, 52, 60, 66 (Figure 14), and 146 (Figure 16), suppressed the superoxide anion generation in fMLP-activated human neutrophils [53]. The structure–activity relationship (SAR) analysis indicated that the C2′-hydroxy and C4′-methoxy enhanced the activity. Furthermore, the chlorinated PEC 127 (Figure 15), reduced the expression of various inflammatory mediators, such as iNOS, COX2 TNF-α, IL-6, IL-1β, and PGE2 in LPS-activated RAW264.7 macrophage. A mechanistic study revealed that compound 127 selectively suppressed phosphorylation of STAT1/3 and ERK1/2 and activation of NF-kB/MAPK/STAT pathways [162]. A recent study reported that the alcohol extracts of agarwood alleviates the occurrence and development of gastric ulcers via inhibiting oxidation and inflammation [163].
Table 21: Inhibitory effect of agarwood compounds on LPS-induced nitric oxide (NO).
|
No.a |
NO inhibition (IC50, μM) |
Ref. |
No.a |
NO inhibition (IC50, μM) |
Ref. |
No.a |
NO inhibition (IC50, μM) |
Ref. |
|
D5 |
7.2 |
31 |
99 |
5.12 |
107 |
192 |
1.6 |
139 |
|
D8 |
7.1 |
31 |
107 |
4.5 |
91 |
193 |
5.8 |
139 |
|
D12 |
3.2 |
30 |
111 |
7.71 |
107 |
194 |
8.1 |
139 |
|
D18 |
12.8 |
31 |
112 |
3.8 |
91 |
195 |
0.6 |
139 |
|
D24 |
14.2 |
30 |
114 |
13.09 |
107 |
196 |
0.7 |
139 |
|
D43 |
2.5 |
30 |
117 |
22.6 |
107 |
203 |
8.0 |
150 |
|
E7 |
53.8 |
31 |
123 |
22.26 |
107 |
204 |
11.4 |
150 |
|
E9 |
9.3 |
31 |
124 |
9.01 |
107 |
207 |
10.5 |
150 |
|
E25 |
12.5 |
30 |
126 |
7.3 |
91 |
208 |
8.8 |
150 |
|
E37 |
17.3 |
30 |
128 |
4.5 |
91 |
210 |
8.6 |
150 |
|
F33 |
8.1 |
32 |
138 |
1.6 |
91 |
213 |
11.5143 |
150 |
|
14 |
6.4 |
91 |
140 |
84 |
113 |
214 |
7.6 |
150 |
|
28 |
5.95 |
107 |
159 |
7.6 |
139 |
215 |
9.3 |
150 |
|
45 |
7.59 |
107 |
160 |
7.4 |
139 |
216 |
7.0 |
150 |
|
51 |
4.6 |
113 |
161 |
2.3 |
139 |
217 |
8.5 |
150 |
|
73 |
7.94 |
107 |
174 |
37.1 |
139 |
218 |
8.5 |
150 |
|
82 |
6.59 |
107 |
188 |
4.3 |
139 |
223 |
12.0 |
150 |
|
85 |
7.94 |
107 |
191 |
1.8 |
139 |
D56 |
5.46 |
26 |
|
D57 |
14.07 |
26 |
D59 |
45.49 |
26 |
B13 |
52.25 |
26 |
|
B14 |
62.57 |
26 |
F43 |
66.0 |
69 |
F44 |
76.8 |
69 |
|
F45 |
62.7 |
69 |
F46 |
18.8 |
69 |
F47 |
72.8 |
69 |
|
G6 |
89.5 |
69 |
G7 |
68.5 |
69 |
G8 |
74.8 |
69 |
|
G9 |
84.3 |
69 |
135a |
3.46 |
121 |
135b |
12.52 |
121 |
|
135c |
> 40 |
121 |
162a |
35.45 |
121 |
aCompound number
3.2. Cytotoxic potential of agarwood compounds/extracts
Agarwood compounds are tested to examine their cytotoxicity in various cancer cell lines such as A549 (human lung), human hepatoma carcinoma cell lines, BEL-7402 and SMMC-7721, Hela (human cervical), K562 (human myeloid leukemia), KB (epidermoid carcinoma), KB-VIN [vincristine (VIN)-resistant KB], MGC-803 (human gastric cancer), OV-90 (human ovarian), breast cancer cell lines, MCF-7 and MDA-MB-231, and SGC-7901 (human gastric). The tested compounds showed weak or moderate cytotoxicity (Table 22).
Table 22: Cytotoxicity potential of agarwood compounds in various cancer cell lines.
|
No.a |
IC50 (cell line) |
Ref. |
|
E11 |
17.85 μg/mL (K562), 21.82 μg/mL (BEL-7402) |
60 |
|
F32 |
33.8 μM (K562) |
68 |
|
F36 |
45.1 μM (K562) |
68 |
|
F38 |
48.6 μM (K562) |
68 |
|
6 |
26.2 μM (A549), 19.2 μM (KB-VIN) |
84 |
|
17 |
47.0 μM (K562), 37.95 μg/mL (SMMC-7721), 35.25 μg/mL (MGC-803), 26.98 μg/mL (OV-90), 33.8 μM (A549), 36.6 μM (KB-VIN), 29.0 μM (MCF-7) |
54,84,81 |
|
19 |
18.1 μM (K562), 20.1 μM (BEL-7402) |
54 |
|
33 |
31.59 μg/mL (SMMC-7721), 33.12 μg/mL (MGC-803), 30.77 μg/mL (OV-90) |
81 |
|
41 |
13.20 μM (K562), 25.91 μM (BEL-7402), 23.51 μM (SGC-7901), 22.00 μM (A549), 30.55 μM (HeLa) |
116 |
|
46 |
45.38 μM (K562), 35.42 μM (SGC-7901), 33.31 μM (A549) |
116 |
|
48 |
22.21 μM (SGC-7901), 8.36 μM (K562), 5.76 μM (BEL-7402) |
54, 114 |
|
55 |
30.01 μg/mL (SMMC-7721), 35.25 μg/mL (MGC-803), 26.98 μg/mL (OV-90) |
81 |
|
56 |
61.31 μM (K562), 28.53 μM (BEL-7402), 17.63 μM (SGC-7901), 49.42 μM (HeLa) |
102 |
|
57 |
37.64 μM (SGC-7901), 27.08 μg/mL (SMMC-7721), 31.17 μg/mL (MGC-803), 33.51 μg/mL (OV-90) |
81, 114 |
|
58 |
21.40 μg/mL (SMMC-7721), 36.42 μg/mL (MGC-803), 35.38 μg/mL (OV-90) |
81 |
|
60 |
43.65 μg/mL, 14. 96 μM (K562) |
106, 102 |
|
61 |
17.8 μM (SGC-7901), 13.9 μM (K562), 31.9 μM (BEL-7402), 25.8 μM (A549), 26.1 μM (KB), 21.9 μM (KB-VIN), 38.1 μM (MDA-MB-231), 28.7 μM (MCF-7) |
54, 84 |
|
62 |
18.82 μg/mL (SMMC-7721), 25.35 μg/mL (MGC-803), 31.60 μg/mL (OV-90) |
81 |
|
65 |
20.01 μg/mL (SMMC-7721), 31.34 μg/mL (MGC-803), 36.64 μg/mL (OV-90) |
81 |
|
78 |
24.85 μg/mL (SMMC-7721), 28.60 μg/mL (MGC-803), 30.40 μg/mL (OV-90) |
81 |
|
81 |
31.06 μg/mL (SMMC-7721), 28.24 μg/mL (MGC-803), 22.54 μg/mL (OV-90) |
81 |
|
84 |
11.83 μM (K562), 25.02 μM (BEL-7402), 29.29 μM (SGC-7901), 44.11 μM (HeLa) |
102 |
|
110 |
35.11 μM (BEL-7402), 32.95 μM (SGC-7901) |
116 |
|
134 |
14.6 mg/mL (SGC-7901) |
134 |
|
136 |
46.1 μM (SGC-7901), 43.8 μM (A549) |
108 |
|
152 |
2.87 μM (K562), 4.75 μM (BEL-7402), 9.91 μM (SGC-7901), 22.43 μM (A549), 13.86 μM (HeLa) |
116 |
|
153 |
40.81 μM (K562), 44.18 μM (BEL-7402) |
109 |
|
175 |
73.5 μM (K562) |
141 |
|
182 |
70.9 μM (K562) |
141 |
|
199 |
44.68 μM (BEL-7402) |
148 |
|
200 |
42.10 μM (BEL-7402) |
148 |
|
213 |
34.20 μM (SGC-7901), 37.99 μM (K562), 36.26 μM (HeLa) |
151 |
|
214 |
11.59 μM (SGC-7901), 22.97 (A549), 10.93 μM (K562), 12.88 μM (HeLa) |
151 |
|
227 |
25.7 μM (BEL-7402), 30.6 μM (HeLa) |
152 |
|
228 |
33.9 μM (K562), 29.9 μM (BEL-7402), 26.7 μM (HeLa), 46.3 μM (A549) |
152 |
|
230 |
24.8 μM (BEL-7402), 30.9 μM (SGC-7901), 17.6 μM (HeLa), 32.0 μM (A549) |
152 |
|
232 |
31.50 μM (SGC-7901), 49.0 μM (A549), 22.12 μM (K562), 30.75 μM (HeLa) |
151 |
|
234 |
39.95 μM (SGC-7901), 28.67 μM (K562), 29.34 μM (HeLa) |
151 |
aCompound number
3.3. Neuronal activity of agarwood compounds/extracts
It is known that agarwood traditionally used as a sedative and analgesic agent [3]. The pharmacological studies reported that agarwood extracts as well as pure compounds showed neuroprotective activity [3,6,12]. For example, the benzene extract of A. malaccensis agarwood reduced spontaneous motility, prolonged hexobarbiturate-induced sleeping time, and decreased rectal temperature, while the petroleum ether, chloroform, or water extracts did showed the similar effect [164]. A bio-guided isolation of a benzene extract yielded the jinkoh-eremol (E3, Figure 7) and agarospirol (B1, Figure 4) are the main active constituents [165,166]. The agarwood essential oil sedated mice through vapor inhalation, and identified the main volatile compounds are benzylacetone, α-gurjunene, and (+)-calarene [167]. The 70% EtOH extract of Vietnamese agarwood induced the expression of brain-derived neurotrophic factor (BDNF) mRNA in rat cultured neuronal cells, and identified the sesquiterpene B5 (Figure 4), is responsible active compound to the observed biological potential [36]. The PEC compound 73 (Figure 14), showed neuroprotective activity in P12 pheochromocytoma, and human U251 glioma cells against glutamate-, and corticosterone-induced neurotoxicity [115]. The alcohol extract of agarwood produced by whole-tree agarwood-inducing technique, and the volatile oil combined with pentobarbital sodium showed hypnotic effect. These tested agents prolonged the sleeping time, and increased the rate of falling asleep in mice [168]. In addition, it is also found that the agarwood essential oil showed sedative-hypnotic effects through the GABAergic system [169]. Agarwood essential oil ameliorates restrain stress-induced anxiety and depression by inhibiting HPA axis hyperactivity [170]. The diterpenoids of agarwood showed antidepressant activity through the synaptic reuptake of serotonin and norepinephrine [171]. A recent study reported that the low molecular weight aromatic compounds (LACs) obtained from the headspace-solid phase microextraction (HS-SPME) of Kyara grade (highest-grade agarwood in Japan), showed strongest sedative activity in mice [172]. Agarwood smoke from Kynam agarwood, showed anti-anxious and anti-depressant effects associated with the increase of serotonin levels in mice [173]. Furthermore, agarwood compounds are reported as as promising therapeutic agents to combat Alzheimer's disease through inhibition of acetylcholinesterase (AChE) activity (Table 23). The AChE inhibitory potential of agarwood compounds are presented in Table 23.
Table 23: Acetylcholinesterase inhibitory activity of agarwood compounds (at 50 µg/mL).
|
No.a |
Inhibition rate(%) |
Ref. |
No.a |
Inhibition rate (%) |
Ref. |
No.a |
Inhibition rate (%) |
Ref. |
|
D22 |
21.2 |
57 |
2 |
24.1 |
80 |
81 |
19.6 |
114 |
|
E15 |
33.3 |
41 |
3 |
14.3 |
80 |
82 |
21.6 |
41 |
|
E16 |
274.8 μM (IC50) |
58 |
6 |
19.3 |
88 |
83 |
41.47 |
120 |
|
E18 |
32.7 |
57 |
8 |
15.8 |
89 |
84 |
33.6 |
88 |
|
E26 |
491.4 μM (IC50) |
58 |
9 |
17.4 |
89 |
87 |
41.27 |
120 |
|
E28 |
158.3 μM (IC50) |
58 |
14 |
38.0 |
111 |
88 |
32.11 |
120 |
|
E29 |
42.9 |
40 |
20 |
11.4 |
41 |
100 |
17.5 |
127 |
|
E33 |
15.2 |
57 |
21 |
20.3 |
83 |
105 |
10.61 |
37 |
|
F17 |
19.5 |
71 |
27 |
16.3 |
80 |
125 |
19.1 |
127 |
|
F19 |
19.4 |
71 |
28 |
23.5 |
88 |
135 |
21.10 |
37 |
|
F21 |
19.1 |
67 |
32 |
10.0 |
80 |
139 |
31.5 |
88 |
|
F22 |
63.1 |
59 |
35 |
17.0 |
80 |
142 |
15.8 |
118 |
|
F23 |
15.0 |
67 |
33 |
14.9 |
80 |
143 |
35.9 |
118 |
|
F26 |
24.1 |
67 |
37 |
26.9 |
80 |
146 |
47.9 |
127 |
|
F30 |
31.0 |
71 |
38 |
25.4 |
83 |
148 |
155.6μM (IC50) |
135 |
|
F35 |
54.2 |
41 |
39 |
24.0 |
41 |
149 |
441.6μM (IC50) |
135 |
|
F40 |
35.3 |
41 |
40 |
10.8 |
100 |
151 |
47.4 |
127 |
|
F41 |
46.2 |
41 |
42 |
10.1 |
88 |
163 |
16.82 |
142 |
|
B6 |
16.35 |
37 |
44 |
12.2 |
114 |
167 |
44.01 |
145 |
|
C1 |
49.9 |
39 |
47 |
15.0 |
80 |
171 |
10.85 |
145 |
|
A1 |
44.5 |
71 |
60 |
22.0 |
106 |
172 |
24.57 |
145 |
|
A2 |
20.8 |
71 |
62 |
35.0 |
109 |
173 |
16.80 |
142 |
|
1 |
18.6 |
80 |
65 |
10.0 |
83 |
185 |
15.66 |
142 |
|
E41 |
48.33 |
50 |
aCompound number
Table 23: Acetylcholinesterase inhibitory activity of agarwood compounds (at 50 µg/mL).
3.4. Anti-diabetic activity of agarwood compounds/extracts
Diabetes mellitus (DM), known as diabetes is a serious, chronic, and complex metabolic disorder [174]. The DM complications affect people both in the developing and developed countries. There are several classes of therapeutic antidiabetic drugs such as sulfonylureas, biguanides, α-glucosidase inhibitors, thiazolidinediones, and non-sulfonylureas secretagogues [174]. The agarwood compounds are reported as α-glucosidase inhibitors. For example, A. filaria sesquiterpenoid, guaianolide (F37) reported as an inhibitor of α-glucosidase with an IC50 value of 253.2 µM [68]. Further, the prezizaane sesquiterpenoids, H1, H4, and H11 (Figure 10), and zizaane sesquiterpenoids, I1 and I3 (Figure 11), are reported to possess the inhibitory effect against α-glucosidase [76]. On the other hand, the PECs compounds 11 and 12 (Figure 14), shown to promote the secretion of adiponectin as PPARγ agonists during adipogenesis in human bone marrow mesenchymal stem cells [82]. The A. sinensis PEC compounds 47, 48 and 65 (Figure 14), reported as inhibitors of α-glucosidase with IC50 values of 90, 50 and 150 µM, respectively [114].
3.5. Antibacterial activities of agarwood compounds/extracts
The sesquiterpenoids and 2-(2-phenylethyl) chromones (PECs) of A. crassna and A. sinensis are examined for their antibacterial activity against Staphylococcus aureus and Ralstonia solanacearum using disk agar diffusion method (Table 24). The PECs of A. sinensis agarwood showed antibacterial activity against S. aureus, and methicillin-resistant S. aureus (MRSA) (Table 24) [99]. The sesquiterpene β-caryophyllene (G5, Figure 9), showed superior antibacterial activity against Gram-positive human pathogenic bacteria than that of Gram-negative bacteria [73]. The extracts of A. crassna agarwood, aqueous, SFE, and SFE with ethanol as the co-solvent, are showed antimicrobial activities against S. aureus and Candida albicans, but are not against Escherichia coli [175].
Table 24: Antibacterial activity (Inhibition zone in mm) of agarwood compounds
|
No.a |
S. aureus |
R. solanacearum |
Ref. |
|
D4 |
20.02 |
11.02 |
52 |
|
D5 |
9.12 |
8.98 |
52 |
|
D8 |
12.90 |
18.20 |
52 |
|
D9 |
14.20 |
10.15 |
52 |
|
D10 |
8.10 |
Not active |
52 |
|
D31 |
12.35 |
16.90 |
40 |
|
6 |
Not active |
6.80 |
89 |
|
54 |
9.10 |
Not active |
88 |
|
56 |
10.01 |
Not active |
88 |
|
145 |
14.95 |
12.09 |
88 |
|
146 |
12.75 |
15.40 |
88 |
|
P4 |
11.20 |
7.81 |
155 |
aCompound number
3.6. Effect of agarwood compounds/extracts on cardiovascular System
It is reported that 50% ethanolic extract of Bawei Chenxiang powder enhanced the hypoxia tolerance of cardiomyocytes [176]. The Tibetan Bawei Chenxiang powder showed a protective effect on the ratmodel of myocardial ischemia [177]. The agarwood alcohol extract ameliorates isoproterenol-induced myocardial ischemia by inhibiting oxidation and apoptosis [178]. The agarwood of A. crassna showed noticeable cardioprotective activities. For example, A. crassna extract reduced simulated ischemia induced cell death in cardiac myoblast cell line, H9c2 [179], as well as isolated adult rat ventricular myocytes [180]. Additionally, the ethyl acetate extract of A. crassna protect the heart from myocardial ischemia/reperfusion injury through attenuation of p38 MAPK phosphorylation [181]. Further, it is also reported the cytoprotective effect of A. crassna extract on actin cytoskeleton organization, in cardiac cell subjected to simulated ischemia [182]. Phosphodiesterases (PDEs) are enzymes that regulate cellular signaling by hydrolysis of intracellular second messengers, cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP) [183]. In cardiovascular tissues, PDE 3A is one of the dominant cAMP-hydrolyzing isozymes, and PDE 3 inhibitors may be used in congestive heart failure [183]. On the other hand, PDE 5 is the major GMP hydrolyzing enzyme in human corpus carvernosal tissue, and PDE 5 inhibitors such as sildenafil have been used to treat erectile dysfunction [183]. A recent study reported that the new FPEC (85a, Fig. 14) and DIPECs 198a‒198d (Figure 18), have considerable activity against PDE (Table 25).79 Additionally, the PECs compounds 19, 20, 47, 48, 56, 60 and 81 (Figure 14), are also reported to have PDE 3A inhibitory activity [104].
Table 25: Effect of agarwood compounds against PDE activity.
|
No.a |
PDE |
IC50 |
Ref. |
|
19 |
PDE 3A |
89.3 µM |
79 |
|
PDE 5A1 |
19.4 µM |
79 |
|
|
48 |
PDE 3A |
4.83 μM |
104 |
|
85a |
PDE 3A |
44.2 µM |
79 |
|
PDE 5A1 |
20.7 µM |
79 |
|
|
198a |
PDE 3A |
> 100 µM |
79 |
|
PDE 5A1 |
4.2 µM |
79 |
|
|
198b |
PDE 3A |
> 100 µM |
79 |
|
PDE 5A1 |
7.9 µM |
79 |
|
|
198c |
PDE 3A |
42.6 µM |
79 |
|
PDE 5A1 |
15.1 µM |
79 |
|
|
198d |
PDE 3A |
> 100 µM |
79 |
|
PDE 5A1 |
4.3 µM |
79 |
aCompound number
3.7. Other activities
The sesquiterpenoid D8 (Figure 6) reported as inhibitor of innate and adaptive immunity through suppressing the STAT signaling pathway [184]. The PECs 18, 65, 75 (Figure 14), and 158 (Fig. 17), showed ABTS•+ radical scavenging activity, with IC50 values of 34.7, 16.5, 12.1 and 12.3 µM, respectively [92]. The A. sinensis PEC compound 136 (Figure 16), suppressed the survival, activation, proliferation, and differentiation of B cells through reduced B-cell activating factor from the tumor necrosis factor family (BAFF) signaling [185]. The PEC compounds 16, 44 and 82 (Fig. 14), showed the tyrosinase inhibitory activity [75,103]. The ethanolic extract of agarwood produced by the whole-tree agarwood-inducing technique, improved the intestinal peristalsis, enhanced gastric emptying, and inhibited gastric ulcer [175]. Additionally, agarwood ethanol extract showed protective effect of intestinal injury induced by fluorouracil (5-FU) through reduced inflammation and, enhanced antioxidant enzymes and Nrf2 signalling [186]. The alcoholic extract of Agar-Wit agarwood alleviate the inflammation and asthma in the asthma mouse model induced by intraperitoneal injection of ovalbumin+Al(OH)3 [187]. The agar wood decotions/infusions traditionally used for alleviating abdominal discomfort, however the gastrointestinal effect on a specific disease is not completely explored yet.
3.8. Biological activities of Excoecaria formosana compounds
Formosins F (N6, Figure 24) showed moderate anti-microbial activity against two strains of Helicobacter pylori (Hp-SS1 and ATCC 43504) with MIC values of 50 and 50 μg/mL, respectively [157]. Compounds N44, and N46–N48 (Figure 24), at a 100 µM concentration showed a 2.97-, 3.17-, 2.73-, 2.63-, 6.57, and 2.62-fold increase in glycine N-methyltransferase (GNMT)-promoter activity, respectively [158].
3.9. Clinical Application of agarwood
The clinical studies indicating that agarwood has therapeutic effect in various diseases, including cardio-cerebrovascular system, urinary system, and respiratory system. The agarwood product Bawei Chenxiang powder (agarwood, nutmeg, jujube, travertine, frankincense, radix aucklandiae, chebula, kapok), showed therapeutic effect in patients with bronchial asthma [188]. Additionally, the Bawei Chenxiang powder showed superior protective effect in the patients with angina pectoris (a coronary heart disease), after continuous administration for four weeks, as compared with the conventional medicine treated patients [189]. In an another study 30 constipation patients are treated with Chenxiang Tongbian powder. The results showed that the total effective rate of Chenxiang Tongbian powder is higher (13.33%) than that of patients treated with polyethylene glycol electrolyte orally, and the symptoms of the patients were significantly relieved after two weeks of treatment [190].
4. EXTRACTION AND ANALYSES OF AGARWOOD
4.1. Extraction of agarwood
In general, the agarwood extraction method depends on the purpose of the extract [191]. The agarwood essential oils are obtained through hydrodistillation, or steam distillation [191]. The chemical constituents of agarwood usually obtained from the solvent extraction, such as acetone, methanol, ethanol and water or supercritical fluid extraction [191]. Various extraction process such as maceration, soxhlet, supercritical fluid, ultrasonic-assisted, microwave-assisted, and high-pressure processing extractions are used to get the desired extract/compounds [191]. Each solvent and/or extraction process produces different extracts in terms of quantity and quality of the constituents [191]. Although water is a cheap solvent and relatively safe, however, aqueous extracts resulted the impurities that makes difficult to isolate the desired compound. Therefore, after the aqueous extraction process, the crude extract was fractionated with hydroalcohol into the desired compounds [191]. This technique is widely applied, especially in the whole process of extraction of the agarwood [191]. Methanol also suitable as an extraction solvent since aqueous methanol was more effective in extracting total sesquiterpenes, 2-(2-phenylethyl)-4H-chromen-4-one derivatives (PECs), and aromatic compounds as compared with water [191]. Alternatively, ethanol is also a suitable solvent for agarwood extraction. It is a non-toxic for human consumption, and widely used for natural products extraction [191]. Most secondary metabolities are dissolved in ethanol except protein, phlegm, pectin, starch and polysaccharide [191].
4.2. Analyses of agarwood
It is difficult to distinguish agarwood quality by observing its morphological characteristics, and the handicrafts of incense are more complicate to identify. Recently, the chemical constituents of agarwood has gained increasing attention due to the agarwood quality is correlate its resin yield and metabolites [72,192]. At present, various methods are established to control the quality of agarwood, including the coloration of chemical reagents, the content of alcohol extracts, the content of agarwood agarotetrol (98, Figure 15), the content of chromone, the HPLC fingerprint of alcohol extract, etc. Using these methods, it is conceivable to clarify the agarwood obtained either from wild or artificial induction methods [193,194]. The Chinese Pharmacopoeia (2020 edition) indicates that the content of the ethanol extract of agarwood resin needs to be more than 10% (w/w, dry weight), and the content of the marker compound, agarotetrol (98, Figure 15) needs to be more than 0.1% [10].
4.2.1. Analyses of agarwood sesquiterpenoids
Several analytical techniques are applied to analyze the agar wood chemical compounds of essential oils, such as electronic nose (Enose), gas chromatography (GC), GC/mass spectrometry (GC/MS), solid phase micro extraction (SPME), GC–flame ionization detector (GC-FID), GC-olfactometry (GC-O), and comprehensive two dimensional GC [191]. Among these, GC/MS, followed by SPME techniques are preferential, which showed promising result in analysing the chemical compounds of agarwood oil [191]. The sesquiterpenoids from the agarwood of A. agallocha and A. malaccensis are identified using the combination of GLC and GC/MS [191]. It is reported that the abundances (percentage of relative peak area measured by GC-MS) of the same compound in high quality oil is more than that of low quality agarwood oil [196]. Further, Ishihara et al., (1993), classified the quality of agarwood oil based on peak area percentage (or abundances) of α-guaiene (F8, Figure 8) with the peak area <0.05% classified as a high quality oil, however the wood oil which is not containing α-guaiene (F8) classified as low quality [65]. Moreover, lower quantity of benzaldehyde (P9, Figure 22) and anisaldehyde (P10, Figure 22) are present in high grade agarwood oil (i.e. Kanankoh), as compared with the low grade (i.e. Jinkoh), agarwood oil [65]. Additionally, the same authors also reported that the quantity of agarwood resin and content of oxygenated sesquiterpenes are comparatively higher in high grade agarwood oil [42]. Table 26 summarizes the component based characteristic of agarwood oil. It is reported that the GC-MS analysis of aromadendrene (F42, Figure 8), showed a positive linier relationship with the agarwood resin yield and quality, and therefore suggests as a marker compound for agarwood grading [72]. The eremophilane-type sesquiterpene, valencene (E39, Figure 7) from A. malaccensis is reported as a marker compound in the grading of agarwood oil [64].
Table 26: Component based characteristic of agarwood oil.
|
Component |
High Quality |
Low Quality |
Ref. |
|
α-guaiene (F8) |
<0.05% |
Not available |
19,65 |
|
Resin content |
high |
low |
197 |
|
Benzaldehyde (P9), anisaldehyde (P10) |
Less amount |
More amount |
65,198 |
|
10-epi-γ-eudesmol (D21), β-agarofuran (D44), α-agarofuran (D49) |
Presence |
Not mentioned |
154 |
|
β-agarofuran (D44) |
Marker compound |
Not mentioned |
199 |
4.2.2. Analyses of agarwood 2-(2-phenylethyl)chromens (PECs)
On the other hand, the PECs compounds are the major fragrance constituents of agarwood, which contributors to the sweet, fruity and long lasting scent of agarwood when it is burn [3,6,12]. The content of agarwood PECs are used to evaluate the grading of agarwood products [86]. Various types of agarwood specific PECs are identified as potential marker for its authentication [25]. These PEC compounds are obtained through solvent extraction methods, but are not extractable using hydrodistillation [200,201]. Structural studies revealed that most of the reported agarwood PEC compounds has the same basic skeleton (MW: 250) and similar substituents, i.e., hydroxy or methoxy or both groups [202]. The determination of PECs in agarwood using GC-MS are relatively limited, as compared with the sesquiterpene constituents. It is reported that agarwood “Kanankoh” oil as a high-grade one, while the “Jinkoh” agarwood is the low quality [42,65,197]. The “Kanankoh” oil contains 66.47 % of PECs and 2-(2-4-methoxy-phenylethyl) chromone, which is higher than the “jinkoh” (1.5%) [196]. The contents of PECs, agarotetrol (98, Figure 15) and isoagarotetrol (103, Figure 15), have positive correlation with the quality of commercial agarwood [203]. An integrated strategy using SHS-GC-MS and UPLC-Q/Tof-MS is used to discriminate the high grade wild Chi-Nan agarwood from A. sinensis, and ordinary agarwood. The results revealed that average contents of 2-(2-phenylethyl) chromones and sesquiterpenes in Chi-Nan agarwood is higher as compared with ordinary agarwood [204]. A recent study reported that the pharmacokinetic results of major PECs in rat plasma using UHPLC with tandem mass spectrometry after oral administration of agarwood ethanol extract [205]. It is interesting to note that HPLC is a superior analytical technique to analyze the agarwood PECs. The details are presented in Table 27.
Table 27: HPLC analyses of agarwood samples.
|
Species |
Sample |
Extraction method |
Column / Temerature |
IVa/DWb |
Mobile phase/ runtime (min) |
FLc (ml/min) / Compounds |
Ref. |
|
Agarwood spp. |
AW |
EtOAc fract. of 95%EtOH |
Gemini RP-C18 (250 ✕ 10, 5 μm) |
5/254 |
CH3OH–H2O (70:30) / 25 |
PECs |
162 |
|
Agarwood spp. |
AW |
alcohol |
Diamonsil C18 (250 × 4.6, 5 μm)/ 32 ᵒC |
10 /252 |
0.7 / |
PECs |
206 |
|
Agarwood spp |
Wild, cultivar |
Phenomenex Luna C18 150×4.6,5mm/31ᵒC |
10 / 252 |
CH3CN - 0.1% HCOOH / 60 |
0.7/ PECs |
207 |
|
|
Agarwood spp |
AW |
95%ethanol |
Diamonsil C18 (4.6 ×250, 5 μm) / 30°C |
5 / 252 |
CH3CN, 0.1% HCOOH |
0.7 / |
208 |
|
A. crassna |
Trunk |
Et2O |
Dionex-Acclaim 120 C18 (250× 4.6, 5 µm)/ 26 ᵒC |
20 /254 |
CH3CN, CH3OOH (99.5:0.5)/ 95 |
0.4 / PECs |
209 |
|
A. sinensis |
AW |
Ultrasonic ext. with Et2O |
Dionex-Acclaim 120 C18(250 ×4.6,5 μm)/ 26°C |
20 / 254 |
CH3CN, HCOOH (99.5:0.5, / 95 |
0.4 mL/min/ PECs |
210 |
|
A. sinensis |
AW |
MeOH ext |
HPLC, QAMS, and UPLC-MS |
PECs |
211 |
||
|
Agarwood spp |
HPLC- L-7100 pump, L-7300 column oven (Hitachi, Ltd.). 5C18MS-II (Cosmosil) column, 4.6 mm × 250 mm; solvent, MeOH–water; FW-1 ml/min, IV-olume, 10 μl; DW-254 nm. |
Agarotetrol |
212 |
||||
AW- agarwood; aIV- Injection volume (in µL); bDW- Detection wavelength (nm); cFL- flow rate; PECs- 2-(2-phenylethyl)chromones
4.2.3. Identification of agarwood adulteration
Due to its high demand and high price, agarwood commercial products are tainted with adulteration and substitution products in order to meet the market demand. Agarwood adulteration happened in different forms, such as painting and covering with oil or making the wood heavier, etc. In general, the impregnation of agarwood with abietic acid or wax to create a resemblance to high-grade agarwood [213]. Powder is the most susceptible agarwood item for adulteration, where it is mixed with healthy (uninfected), wood. Two types of fake agarwood have been described; (i) low quality agarwood painted with small layer of shavings mixed with wax and other material; and (ii) “Black Magic Wood” which refers to low quality agarwood impregnated with agarwood oil and alcohol [214]. Iron shavings and carbon powder from spent batteries are also used to increase the weight and create resemblance to high grade agarwood [51]. In Taiwan market, inferior quality of agarwood has been increasingly mis-classified and substituted as the top-grade agarwood. On the other hand, agarwood oil is adulterated either with ‘lodh’ oil, kerosene, other coloured oils, a mixture of other chemicals that gives the aroma of agarwood [51]. In this connection, various synthetic agarwood compounds are developed [118,212]. However, these are used to produce poor-quality fragrances as no synthetic substitutes are available for high-grade fragrances due to the complexity of natural agarwood composition [51].
Various PECs are specific in agarwood. The DART-TOFMS characteristic fragmentation behavior of PECs is applied for their accurate identification in agarwood [215]. The presence of the diagnostic TPEC ions at m/z 349.129 or at m/z 319.118, are characteristic of the PECs [215]. Interestingly, cultivated agarwood and wild agarwood samples showed differences in PECs, which helped to distinguish the wild agarwood from cultivated one [216]. The characteristic fragmentation behaviors of FPECs, and cleavage of the CH2–CH2 bond between the chromone moiety and phenyl moiety are used to calculate the number of methoxy or hydroxy groups, which enabled the identification of FPECs [202]. Further, the characteristic fragmentation behaviors of DPECs, EPECs and TPECs are analyzed using LC-MS without databases or reference standards [209]. A recednt study reported an integrated method of FT-NIR, GC-MS and UHPLC-Q-Exactive Orbitrap/MS, to identify the chemical variation between wild and cultivated agarwood. This novel method identified eight key marker compounds, including flidersia type (FPECs)-sesquiterpenes and TPECs, which are putatively distinguish between wild and cultivated agarwood [217]. Tian et al., developed an UHPLC-TOF-MS method to compare the chemical composition and the bioactivities of wild and artificial agarwood [218]. The Liquid extraction surface analysis mass spectrometry (LESA-MS) is applied in the direct qualitative analysis of agarwood from different sources [219]. During this study, a characteristic 2-(2-phenylethyl)chromone compound (m/z 319.1) is treated as a marker to identify agarwood and its counterfeits [219]. The headspace GC-MS is used to to distinguish the sesquiterpenes compounds between the high-quality agarwood Kynam, and cultivated grafting Kynam [220].
CONCLUSIONS / FUTURE PROSPECTS
The major compounds of agarwood apecies are sesquiterpenoids, and 2-(2-phenylethyl)chromone. These are diverse and complex, and are influenced by agarwood plant species, formation process, collection time, extraction process, and analytical approach etc. Further studies are required to understand the agarwood resin composition, and to identify specific marker compounds of different agarwood plant species through metabolomics approach. The agarwood formation mechanism is not completely revealed; therefore, studies are required to understand the essential compounds biosynthesis mechanism of agarwood resin. Although, various pharmalogical activities are reported for the pure compounds/extracts, however, the specific active substances and pharmacological action mechanisms are not been confirmed. Therefore, systematic in vitro and in vivo studies are required for the identification of effective components of agarwood and explore their action mechanism. The active compounds can be used as a template to develop novel therapeutic agents. The relationship between the active compounds and their pharmacokinetics need to be identified to develop their clinical use. Further systematic analytical methods are required to chemically distinguish the different kinds of agarwood. Studies are required to establish an accurate and rapid identification of fake and shoddy products in commercial products.
REFERENCES
- Wang Y, Hussain M, Jiang Z, Zhaohong W, Jing G et al. (2021). Aquilaria Species (Thymelaeaceae) distribution, volatile and non-nolatile phytochemicals, pharmacological uses, agarwood grading system, and induction methods. Molecules. 26(24):7708.
- Tan CS, Isa NM, Ismail I, Ismanizan Ismail, Z Zainal. (2019). Agarwood induction: current developments and future perspectives. Front Plant Sci. 10:122.
- Hashim YZ, Kerr PG, Abbas P, HM Salleh. (2016). Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J Ethnopharmacol. 189:331‒360.
- Naziz PS, Das R, Sen S. (2019). The scent of stress: Evidence from the unique fragrance of agarwood. Front Plant Sci.10:840.
- Azren PD, Lee SY, Emang D, R Mohamed. (2019). History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: a review. J For Res. 30:1–11.
- Gao M, Han X, Sun Y, Chenc H, Yang Y et al. (2019). Overview of sesquiterpenes and chromones of agarwood originating from four main species of the genus Aquilaria. RSC Adv. 9:4113‒4130.
- Li GD, Rao PY, Guo JL, Zhang YH. (2019). The complete chloroplast genome of a critically endangered agarwood tree, Aquilaria crassna (Thymelaeaceae). Mitochondrial DNA Part B. 4(1):810‒1811.
- López-Sampson A, Page T. (2018). History of use and trade of agarwood. Econ Bot. 72:107–129.
- Adhikari SR, Pokhrel K, Baral S.D. (2021). Economic value of agarwood and its prospects of cultivation. Int J Appl Sci Biotechnol. 9(1):23‒31.
- Chinese Pharmacopoeia. National Pharmacopoeia Committee. 2020. http://wp.chp.org.cn/front/chpint/en/
- Yadav VK, Choudhary N, Heena Khan S, Areeba K. (2020). Incense and incense sticks: types, components, origin and their religious beliefs and importance among different religions. J Bio Innov. 9:1420‒1439.
- Li W, Chen HQ, Wang H, Wen LM, Hao F D. (2021). Natural products in agarwood and Aquilaria plants: chemistry, biological activities and biosynthesis. Nat Prod Rep. 38(3):528‒565.
- Rasool S, Mohamed R. (2016). Understanding agarwood formation and its challenges, in Agarwood: Science Behind the Fragrance, ed. R. Mohamed. (Berlin:Springer). 39–56.
- Cui J, Guo S, Fu S, Pei GX. (2013). Effects of inoculating fungi on agilawood formation in Aquilaria sinensis. Chin Sci Bull 58(26):3280–3287.
- Siburian RH, Siregar UJ, Siregar IZ, Edwin S. (2015). Identification of morphological characters of Aquilaria microcarpa in the interaction with Fusarium solani. Int J Sci Basic Appl Res. 20(2):119–128.
- Nagajothi SM, Parthiban KT, Kanna SM, Loganathan K. (2016). Fungal microbes associated with agarwood formation. Am J Plant Sci. 7(10):1445–1452.
- Mohamed R, Jong PL, Kamziah AK. (2014). Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J For Res. 25:201–204.
- Liu Y, Chen H, Yang Y, Zheng Z, Jianhe W, et al. (2013). Whole-tree agarwood-inducing technique: an efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules. 18(3):3086–3106.
- Zhang XL, Liu YY, Wei JH, Yun Y, Zheng Z, et al. (2012). Production of high-quality agarwood in Aquilaria sinensis trees via whole-tree agarwood-induction technology. Chin Chem Lett. 23:727–730.
- Wu ZQ, Liu S, Li JF, MC Li. (2017). Analysis of gene expression and quality of agarwood using Agar-bit in Aquilaria sinensis. J Trop For Sci. 29(3):380–388.
- Fazila KN, Halim KHK. (2012). Effects of soaking on yield and quality of agarwood oil. J Trop Fores Sci. 24(4):557–564.
- Chen HQ, Wei JH, Yang JS, Zheng Z, Yun Y, et al. (2012). Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem Biodivers. 9(2):236–250.
- Varma RK, Maheshwari ML, Bhattacharyya SC. (1965). Terpenoids—LXII: The constitution of agarospirol, a sesquiterpenoid with a new skeleton. Tetrahedron. 21(1):115–138.
- Naf R, Velluz A, Brauchli R, Thommen W. (1995). Agarwood oil (Aquilaria agallocha Roxb.). Its composition and eight new valencane-, eremophilane- and vetispirane- derivatives. Flavour Fragr J. 10(3):147–152.
- Naef R. (2011). The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: a review. Flavour Fragr J. 26(2):73–89.
- Xie Y, Song L, Li C, Yang Y, Zhang S, et al. (2021). Eudesmane-type and agarospirane-type sesquiterpenes from agarwood of Aquilaria agallocha. Phytochemistry. 192:112920.
- Nat R, Velluz A, Thommen W, Brauchli R,Sigwart C, et al. (1993). New compounds identified in agarwood (Aquilaria agallocha Roxb.). Flavour Fragr J. 8(6):307–313.
- Nakanishi T, Yamagata E, Yoneda K. (1983). Jinkoh-eremol and jinkohol II, two new sesquiterpene alcohols from agarwood. J Chem Soc Perkin Trans. 1:601–604.
- Lin LD, Qi SY. (2000). Triterpenoid from Chinese eaglewood (Aquilaria sinensis). Chin Trad Herb Drugs. 31:89–90.
- Yu ZX, Wang CH, Chen DL, Liu YY, Wei JH. (2019). Anti-inflammatory sesquiterpenes from agarwood produced via whole-tree agarwood-inducing technique of Aquilaria sinensis. China J Chin Mater Med. 44(19):4196–4202.
- Huo HX, Zhu ZX, Dao RP, Li YT, Huang Z, et al. (2015). Anti-neuroinflammatory sesquiterpenes from Chinese eaglewood. Fitoterapia. 106:115–121.
- Zhao H, Peng QH, Han ZZ, Yang Li, Wang Z. (2016). Three new sesquiterpenoids and one new sesquiterpenoid derivative from Chinese eaglewood. Molecules. 21(3):281.
- Nakanishi T, Yamagata E, Yoneda K, Nagashima T, Kawasaki I. et al. (1984). Three fragrant sesquiterpenes of agarwood. Phytochemistry. 23(9):2066–2067.
- Wu B, Kwon SW, Hwang GS, Park JH. (2012). Eight new 2-(2-phenylethyl)chromone (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv Chim Acta. 95(9):1657–1665.
- Ishihara M, Tsuneya T, Shiga M, Uneyama K. (1991). Three sesquiterpenes from agarwood. Phytochemistry. 30(2):563–566.
- Ueda JY, Imamura L, Tezuka Y, Tran QL, Masaaki Tsuda et al. (2006). New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorg Med Chem. 14(10):3571–3574.
- Xiang P, Zeng YB, Mei WL, et al. (2017). Chemical constituents and bioactivities of Aquilaria lignum resinatum by artificial holing method. J Chin Med Mater 40:2339–2343.
- Tian H, Wang H, Yang L, Gai CJ, Dong WH, et al. (2020). Two new sesquiterpenoids from agarwood originated from Aquilaria sp. J Asian Nat Prod Res. 22(7):626–631.
- Li W, Mei WL, Dong WH, Hong CC, Juan GC, et al. (2019). Study on chemical constituents of Chinese agarwood 'Qi-Nan'. J Trop Subtrop Bot. 27(2):196–202.
- Wang HN, Dong WH, Huang SZ, Li W, Kong FD, et al. (2016). Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 114:7–11.
- Shao H, Mei WL, Kong FD, Dong WH, Gai CJ, et al. (2016). Sesquiterpenes of agarwood from Gyrinops salicifolia. Fitoterapia. 113:182–187.
- Ishihara M, Tsuneya T, Uneyama K. (1993). Fragrant sesquiterpenes from agarwood. Phytochemistry 33(5):1147–1155.
- Naf R, Velluz A, Busset N, Gaudin JM. (1992). New nor-sesquiterpenoids with 10-epi-eudesmane skeleton from agarwood (Aquilaria agallocha Roxb.). Flavour Fragra J. 7(6):295–298.
- Kristanti AN, Tanjung M, Aminah NS. (2018). Review: secondary metabolites of Aquilaria, a Thymelaeaceae genus. Mini-Rev Org Chem. 15(1):36–55.
- Maheshwari ML, Jain TC, Bates RB, Bhattacharyya SC. (1963). Terpenoids—XLI: Structure and absolute configuration of α-agarofuran, ⨿-agarofuran and dihydroagarofuran. Tetrahedron. 19(6):1079–1090.
- Maheshwari ML, Varma RK, Bhattacharyya SC. (1963). Terpenoids—XLVII: Structure and absolute configuration of norketoagarofuran, 4-hydroxydihydroagarofuran, 3,4-dihydroxydihydroagarofuran and conversion of β-agarofuran to α-agarofuran. Tetrahedron 19(10):1519–1525.
- Yang JS, Chen YW. (1986). Chemical constituents of Aquilaria sinensis (Lour.) Gilg. II. Isolation and structure of baimuxinol and dehydrobaimuxinol. Acta Pharm. Sin 21(7):516–520.
- Yang JS, Wang YL, Su YL. (1989). Chemical constituents of Aquilaria sinensis (Lour.) Gilg. III. Elucidation of the structure of isobaimuxinol and isolation and identification of the constituents of lower boiling point fraction of the volatile oil. Acta Pharm Sin 24:264–268.
- Yang JS, Wang YL, Su Y. (1992). Baimuxifuranic acid, a new sesquiterpene from the volatile oil of Aquilaria sinensis (Lour.) Gilg. Chin Chem Lett. 3:983–984.
- Li W, Yang YL, Yang L, Wanga H, Dong WH, et al. (2020). New sesquiterpenoids bearing 11-methyl ester group of agarwood. Fitoterapia. 143:104557.
- Barden A, Anak NA, Mulliken T, Son M. (2000). Heart of the matter: agarwood use and trade and CITES implementation for Aquilaria malaccensis. TRAFFIC, 52:2000.
- Li W, Cai CH, Guo ZK, Wang H, Zuo WJ, et al. (2015). Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia. 100:44–49.
- Wang SL, Liao HR, Cheng MJ, Shu CW. (2018). Four new 2-(2-Phenylethyl)-4H-chromen-4-one derivatives from the resinous wood of Aquilaria sinensis and their inhibitory activities on neutrophil pro-Inflammatory responses. Planta Med. 84(18):1340–1347.
- Shao H, Mei WL, Dong WH, Gai CJ, Li W, et al. (2016). 2-(2-phenylethyl)chromone derivatives of agarwood originating from Gyrinops salicifolia. Molecules. 21(10):1313.
- Ishihara M, Tsuneya T, Uneyama K. (1991). Guaiane sesquiterpenes from agarwood. Phytochemistry 30(10):3343–3347.
- Kuang TD, Chen HD, Li W, et al. (2017). Chemical constituents of agarwood induced by artificial holing. China J Chin Mater Med. 42:4618–4623.
- Kang KX, Dai HF, Wang P. (2017). Sesquiterpenoids of agarwood from Aquilaria crassna. Chin Tradit Herb Drugs. 48(24):4601–4607.
- Yang DL, Wang H, Guo ZK, Liab W, Mei WL, et al. (2014). Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem Lett. 8:121–125.
- Xu JF, Zhu LF, Lu BY, Liu Ct. (1988). Study on the chemical constituents of essential oils of Aquilaria sinensis. Acta Bot Sin. 30(6):635–638.
- Chen HQ, Guo FJ, Cai CH, et al. (2019). Study on sesquiterpenoids in pseudo-agarwood of willow leaves. China J Chin Mater Med 44:2274–2277.
- Li W, Liao G, Dong WH, Kong FD, Wang P, et al. (2016). Sesquiterpenoids from Chinese agarwood induced by artificial holing. Molecules 21(3):274.
- Wang SL, Tsai YC, Fu SL, Cheng MJ, Chung MI, et al. (2018). 2-(2-phenylethyl)-4H-chromen-4-one derivatives from the resinous wood of Aquilaria sinensis with anti-inflammatory effects in LPS-induced macrophages. Molecules 23(2):289.
- Wang P, Kang KX, Kong FD, et al. (2018). A new sesquiterpene in Kolasna agarwood. Chin Tradit Herb Drugs 49:4480–4483.
- Jayachandran K, Sekar I, Parthiban KT, Damodarasamy A and Suresh KK. (2015). Analysis of different grades of agarwood (Aquilaria malaccensis Lamk.) oil through GC-MS. Ind J Nat Prod Res. 5(1):44‒47.
- Ishihara M, Tsuneya T, Uneyama K. (1993). Components of the agarwood smoke on heating. J Essent Oil Res. 5:419–423.
- Ma CT, Eom T, Cho E, Wu B, Kim TR, et al. (2017). Aquilanols A and B, macrocyclic humulene-type sesquiterpenoids from the agarwood of Aquilaria malaccensis. J Nat Prod. 80(11):3043–3048.
- Yang DL, Li W, Dong WH, Wang J, Mei WL, et al. (2016). Five new 5,11-epoxyguaiane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Fitoterapia. 112:191–196.
- Mi CN, Mei WL, Wang H, Yang L, Dong WH, et al. (2019). Four new guaiane sesquiterpenoids from agarwood of Aquilaria filaria. Fitoterapia. 135:79–84.
- Ma CT, Ly TL, Le THV, Tran TVA, Kwon SW, et al. (2021). Sesquiterpene derivatives from the agarwood of Aquilaria malaccensis and their anti-inflammatory effects on NO production of macrophage RAW 264.7 cells. Phytochemistry. 183:112630.
- Ishihara M, Masatsugu Y, Uneyama K. (1992). Preparation of (−)-guaia-1(10),11-dien-15,2-olide and (−)-2α-hydroxyguaia-1(10),11-dien-15-oic acid, fragrant sesquiterpenes in agarwood (Aquilaria agallocha Roxb.). Tetrahedron 48(4):10265–10276.
- Yang DL, Wang J, Li W, Dong W, Mei W, et al. (2016). New guaiane and acorane sesquiterpenes in high quality agarwood Qi-Nan from Aquilaria sinensis. Phytochem Lett. 17:94–99.
- Pasaribu GT, Waluyo TK, Pari G. (2015). Analysis of chemical compounds distinguisher for agarwood qualities. Indonesian. J For Res. 2(1):1-7.
- Dahham SS, Tabana YM, Iqbal MA, Ahamed MBK, Ezzat MO, et al. (2015). The anticancer, antioxidant and antimicrobial properties of the esquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 20(7):11808–11829.
- Nakanishi T, Yamagata E, Yoneda K, Miura I. (1981). Jinkohol, a prezizane sesquiterpene alcohol from agarwood. Phytochemistry. 20:1597–1599.
- Yang L, Yang YL, Dong WH, Li W, Wang P, et al. (2019). Sesquiterpenoids and 2-(2-phenylethyl)chromones respectively acting as α-glucosidase and tyrosinase inhibitors from agarwood of an Aquilaria plant. J Enzyme Inhib Med Chem. 34(1):853–862.
- Yang YL, Li W, Wang H, Yanga L, Yuan JZ, et al. (2019). New tricyclic prezizaane sesquiterpenoids from agarwood. Fitoterapia 138:104301.
- Ma CT, Cho E, Nguyen HT, Hong WT, Le V, et al. (2020). Malacinones A and B, two novel sesquiterpenoids with 6/6/5 tricyclic ring system from the agarwood of Aquilaria malaccensis. Tetrahedron Lett. 61(1):151355.
- Ibrahim SRM, Mohamed GA. (2015). Natural occurring 2-(2-phenylethyl) chromones, structure elucidation and biological activities. Nat Prod Res 29(16):1489‒1520.
- Shibata S, Sugiyama T, Uekusa Y, Masui R, Narukawa Y, et al. (2020). Five new 2-(2-phenylethyl)chromone derivatives from agarwood. J Nat Med 74(3):561‒570.
- Yang DL, Mei WL, Zeng YB, Guo ZK, Zhao YX, et al. (2013). 2-(2-phenylethyl)chromone derivatives in Chinese agarwood Qi-Nan from Aquilaria sinensis. Planta Med 79(14):1329–1334.
- Liu YY, Chen DL, Yu ZX, Hong WC, Feng J, et al. (2020). New 2-(2-phenylethyl)chromone derivatives from agarwood and their inhibitory effects on tumor cells. Nat Prod Res. 34(12):1721–1727.
- Ahn S, Ma CT, Choi JM, An S, Lee M, et al. (2019). Adiponectin-secretion-promoting phenylethylchromones from the agarwood of Aquilaria malaccensis. J Nat Prod. 82(2):259–264.
- Yang DL, Wang H, Guo ZK, Dong WH, Mei WL, et al. (2014). A new 2-(2-phenylethyl)chromone derivative in Chinese agarwood Qi-Nan from Aquilaria sinensis. J Asian Nat Prod Res 169(7):770–776.
- Suzuki A, Miyake K, Saito Y, Rasyid FA, Tokuda H, et al. (2017). Phenylethylchromones with in vitro antitumor promoting activity from Aquilaria filaria. Planta Med. 83(3-4):300–305.
- Konishi T, Konoshima T, Shimada Y, Kiyosawa S. (2002). Six new 2-(2-phenylethyl)chromones from agarwood. Chem Pharm Bull. 50(3):419–422.
- Shimada Y, Tominaga T, Konishi T, Kiyosawa S. (1982). Studies on the agarwood (Jinko). I. Structures of 2-(2-phenylethyl) chromone derivatives. Chem Pharm Bull 30(10):3791–3795.
- Mei WL, Liu J, Li XN, Dai HF. (2010). Study on the chemical constituents from Chinese eaglewood in Hainan. J Trop Subtrop Bot. 18:573–576.
- Li W, Cai CH, Dong WH, Guo ZK, Wang H, et al. (2014). 2-(2-phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia. 98:117–123.
- Wang HN, Mei WL, Dong WH, Kong FD, Li W, et al. (2018). Two new 2-(2-Hydroxy-2-phenylethyl)chromens from agarwood originating from Aquilaria crassna. J Asian Nat Prod Res. 20(2):122–127.
- Sugiyama T, Narukawa Y, Shibata S, Masui R, Kiuchi F, et al. (2018). Three new 5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone derivatives enantiomeric to agarotetrol from agarwood. J Nat Med. 72(3):667–674.
- Huo HX, Gu YF, Sun H, Zhanga YF, WJ Liu, et al. (2017). Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia. 118:49–55.
- Li CG, Pan L, Han ZZ, Xie YQ, Hu HJ, et al. (2020). Antioxidative 2-(2-phenylethyl)chromones in Chinese eaglewood from Aquilaria sinensis. J Asian Nat Prod Res. 22(7):639–646.
- Yu ZX, Wang CH, Zheng W, et al. (2019). Separation and anti-inflammatory effect of agarwood chromones produced by whole body knotting technology. Chin Pharm J 54:1945–1950.
- Hashimoto K, Nakahara S, Inoue T, Sumida Y, Takahashi M, et al. (1985). A new chromone from agarwood and pyrolysis products of chromone derivatives. Chem Pharm Bull. 33(11):5088–5091.
- Yagura T, Michiho I, Fumiyuki K, Honda G, Shimada Y, et al. (2003). Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull 51(5):560–564.
- Wu B, Lee JG, Lin CJ, Jia SD, Kwon SW, et al. (2012). Sesquiterpenoids and 2-(2-phenylethyl)-4H-chromen-4-one (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv Chim Acta 95(4):636–642.
- Alkhathlan HZ, Alhazimi HM, Aldhalaan FS, Mousa AA , et al. (2005). Three 2-(2-phenylethyl)chromones and two terpenes from agarwood. Nat Prod Res. 19(4):367–372.
- Nakanishi T, Inada A, Nishi M. Yamagata and K. Yoneda. (1986). A new and a known derivatives of 2-(2-phenylethyl) chromone from a kind of agarwood (“Kanankoh,” In Japanese) originating from Aquilaria agallocha. J Nat Prod. 49(6):1106–1108.
- Lei ZD, Liu DL, Zhao YM, Gao XX. (2018). A new 2-(2-phenylethyl)chromone from Aquilaria sinensis. Chem Nat Compd. 54(1):30–33.
- Iwagoe K, Konishi T, Kiyosawa S, Shimada, Y, Miyahara, K, et al. (1988). Studies on the agalwood (Jinko). VII.: structures of phenylethyl-chromone derivatives AH7, AH8 and AH9. Chem Pharm Bull. 36(7):2417–2422.
- Yang JS, Wang YL, Su YL. (1990). Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. V. Isolation and characterization of three 2-(2-phenylethyl) chromone derivatives. Acta Pharm Sin 25(3):186–190.
- Zhao YM, Yang L, Li W, Juan GC, Li MW, et al. (2019). 2-(2-phenylethyl) chromones from the agarwood of Aquilaria crassna. Nat Prod Res Dev 31(5):821–826.
- Zhao YM, Yang L, Dong WH, LiW, Chen HQ, et al. (2019). Three new 2-(2-phenylethyl)chromone derivatives from agarwood of Aquilaria crassna Pierre ex Lecomte (Thymelaeaceae) in Laos. Phytochem Lett 32:134–137.
- Sugiyama T, Narukawa Y, Shibata S, Masui R, Kiuchi F, et al. (2016). New 2-(2-phenylethyl)chromone derivatives and inhibitors of phosphodiesterase (PDE) 3A from agarwood. Nat Prod Commun 11(6):795–797.
- Yang L, Guo PY, Guo SX, et al. (2016). 1 new 2-(2-phenethyl) chromone in domestic agarwood. Chin Tradit Herb Drugs 47:4137–4140.
- Xia LL, Li W, Mei WL, Yang L, CC Hong et al. (2019). Chemical constituents of agarwood originating from Aquilaria crassna in Cambodia. Guihaia 39(4):540–547.
- Chen D, Xu ZR, Chai XY, Zeng KW, Jia Y et al. (2012). Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur J Org Chem. 2012:5389–5397.
- Xia L, Li W, Mei WL, Wang, H, Dong WH, et al. (2019). 1 new 2-(2-phenethyl) chromone in korasna agarwood Cambodia. Chin Tradit Herb Drugs 50:4863–4866.
- Xia LL, Li W, Wang H, Dong WH, CH Cai et al. (2019). One new 2-(2-phenylethyl)chromone derivative from agarwood of Aquilaria crassna in Cambodia. Phytochem Lett 31:69–72.
- Gao YH, Liu JM, Lu HX, Wei ZX. (2012). Two new 2-(2-phenylethyl)chromen-4-ones from Aquilaria sinensis (Lour.) Gilg. Helv Chim Acta 95(6):951–954.
- Shao H, Mei WL, Li W, et al. (2015). Study on the chemical constituents of agarwood produced from willow-like agarwood. Nat Prod Res Dev 27:2046–2049.
- Yang JS, Wang YL, Su YL. (1989). Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl)chromen-4-one derivatives. Acta Pharm Sin. 24(9):678–683.
- Liu YT, Chen DL, Wei JH, Feng J, Zhang Z, et al. (2016). Four new 2-(2-phenylethyl)chromone derivatives from Chinese agarwood produced via the whole-tree agarwood-inducing technique. Molecules. 21(11):1433.
- Liao G, Mei WL, Dong WH, Li W, Wang P et al. (2016). 2-(2-phenylethyl)chromone derivatives in artificial agarwood from Aquilaria sinensis. Fitoterapia. 110:38–43.
- Yang L, Qiao LR, Xie D, Yuan Y, N Chen et al. (2012). 2-(2-phenylethyl)chromones from Chinese eaglewood. Phytochemistry. 76:92–97.
- Tian H, Dong WH, Wang H, Wei L, Li Y, et al. (2019). 2-(2-phenylethyl) chromone derivatives of an agarwood from abroad. Chin J Trop Crops. 40(8):1626–1632.
- Liu JM, Gao YH, Xu HH, ZQ Xu. (2007). Chemical constituents of Lignum Aquilariae resinatum(Ⅱ). Chin Tradit Herb Drugs 38(8):1138–1140.
- Gao YH, Liu JM, Xu HH. (2005). Green chemistry problems in the synthesis of aroma raw materials I. Catalytic synthesis of aroma raw materials and their intermediates. Chin J Org Chem 25(1):125-134.
- Shao H, Mei WL, Kong FD, Dong WH, Li W, et al. (2017). A new 2-(2-phenylethyl)chromone glycoside in Chinese agarwood Qi-Nan from Aquilaria sinensis. J Asian Nat Prod Res 19(1):42–46.
- Kuang TD, Chen HQ, Kong FD, Cai CH, L Yang et al. (2018). Three new 2-(2-phenylethyl)chromone derivatives from artificial holing agarwood of Aquilaria sinensis. Phytochem Lett 26:96–100.
- Yu Z, Wang C, Zheng W, Chen D, Liu Y, et al. (2020). Anti-inflammatory 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from agarwood of Aquilaria sinensis. Bioorg Chem 99:103789.
- Zhao YM, Yang L, Kong FD, Dong WH, Li W, et al. (2021). Three new 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones and one new dimeric 2-(2-phenylethyl)chromone from agarwood of Aquilaria crassna Pierre ex Lecomte in Laos. Nat Prod Res 35(14):2295‒2302.
- Dai HF, Liu J, Han Z, Zeng YB, Wang H, et al. (2010). Two new 2-(2-phenylethyl)chromones from Chinese eaglewood. J Asian Nat Prod Res 12:134–137.
- Yoshii E, Koizumi T, Oribe T, Takeuchi F and Kubo K. (1978). The structure of agarotetrol, a novel highly oxygenated chromone from agarwood(jinko). Tetrahedron Lett. 19(41):3921–3924.
- Shimada Y, Konishi T, Kiyosawa S, Nishi M, Miyahara K, et al. (1986). Studies on the agalwood (Jinko). IV.: structures of 2-(2-phenylethyl)chromone derivatives, agarotetrol and isoagarotetrol. Chem Pharm Bull 34(7):2766–2773.
- Shao H, Kong FD, Wang H, Mei WL and Dai HF. (2017). Qinanmer, a new compound from Chinese agarwood Qi-Nan originating from Aquilaria sinensis. J Asian Nat Prod Res 19(9):935–940.
- Liao G, Mei WL, Kong FD, Li W, Yua J, et al. (2017). 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry 139:98–108.
- Konishi T, Iwagoe K, Sugimoto A, Kiyosawa S, Fujiwara Y, et al. (1991). Studies on agalwood (Jinko). X. structures of 2-(2-phenylethyl) chromone derivatives. Chem Pharm Bull 39:207–209.
- Konishi T, Kiyosawa S, Shimada Y, et al. (1989). The structure of AH16, new tetrahydroxy-2-(2-phenylethyl)chromone from agalwood. Chem Pharm Bull 37:1428–1430.
- Shimada Y, Konishi T, Kiyosawa S. (1986). Studies on the agalwood (Jinko). VI.: structures of three 2-(2-phenylethyl)-5, 6, 7, 8-tetrahydrochromone derivatives, AH1A, AH2a and AH2b. Chem Pharm Bull 34:3033–3037.
- Konishi T, Sugimoto A, Kiyosawa S, Fujiwara Y. (1992). Studies on the agalwood "Jinko." XII. structures of pentahydroxy-2-(2-phenylethy)chromone derivatives. Chem Pharm Bull 40(3):778–779.
- Zheng KM, Zhang JQ, Shen PN. (2011). Preparative isolation and purification of agarotetrol and 4′-methoxyagarotetrol from Aquilaria sinensis (Lour.) Gilg by high-speed counter-current chromatography. Chin Tradit Pat Med 33:96–99.
- Dai HF, Liu J, Zeng YB, Han Z, Wang H, et al. (2009). A new 2-(2-phenylethyl)chromone from Chinese eaglewood. Molecules. 14(12):5165–5168.
- Liu J, Wu J, Zhao YX, Deng YY, WL Mei, et al. (2008). A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin Chem Lett 19(8):934–936.
- Li W, Liao G, Wang H, Dong WH, Yang L, et al. (2019). Five new epoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from Chinese agarwood by artificial holing. Fitoterapia 134:182–187.
- Yagura T, Shibayama N, Michiho I, Kiuchi F, Honda G. (2005). Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett 46:4395–4398.
- Dong WH, Wang H, Guo FJ, WL Mei HQ Chen, et al. (2019). Three new 2-(2-phenylethyl)chromone derivatives of agarwood originated from Gyrinops salicifolia. Molecules 24(3):576.
- Li JT, Kuang TD, Chen HQ, Yang L, Wang H, et al. (2021). New 2-(2-phenylethyl)chromone derivatives from agarwood originating from Aquilaria sinensis. J Asian Nat Prod Res 27:1‒8.
- Huo HX, Gu YF, Zhu ZX, Zhang YF, Chen XN, et al. (2019). LC-MS-guided isolation of anti-inflammatory 2-(2-phenylethyl)chromone dimers from Chinese agarwood (Aquilaria sinensis). Phytochemistry 158:46–55.
- Iwagoe K, Konishi T, Kiyosawa S, Shimada, Y, Miyahara, K, et al. (1986). The structures of AH10 and AH11 nobel biphenylethylchromones from agalwood. Chem Pharm Bull 34(11):4889–4891.
- Yang Y, Mei WL, Kong FD, Chen HQ, W Li et al. (2017). Four new bi-2-(2-phenylethyl)chromone derivatives of agarwood from Aquilaria crassna. Fitoterapia 119:20–25.
- Kuang TD, Chen HQ, Wang H, Kong FD, Cai CH, et al. (2019). UPLC-MS-guided isolation of single ether linkage dimeric 2-(2-phenylethyl)chromones from Aquilaria sinensis. RSC Adv 9(30):17025–17034.
- Iwagoe K, Kodama S, Konishi T, Kiyosawa S, Fujiwara Y, et al. (1987). The structures of AH15 and AH18, new bi- and tri-phenylethylchromones from agalwood. Chem Pharm Bull. 35(11):4680–4682.
- Iwagoe K, Kakae T, Konishi T, Kiyosawa S, Fujiwara Y, et al. (1989). Studies on the agalwood (Jinko).VIII.: structures of bi-phenylethylchromone derivatives. Chem Pharm Bull 37:124–128.
- Xiang P, Mei WL, Chen HQ, Kong F, Wang H, et al. (2017). Four new bi-phenylethylchromones from artificial agarwood. Fitoterapia 120:61–66.
- Xiang P, Chen H, Cai C, Wang H, Zhou L, et al. (2020). Six new dimeric 2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis. Fitoterapia 142:104542.
- Xiang P, Dong WH, Cai CH, Li W, Zhou LM, et al. (2021). Three new dimeric 2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis. Nat Prod Res 35(21):3592‒3598.
- Li W, Yang Y, Dong WH, Wang H, Kong F, et al. (2019). Dimeric 2-(2-phenylethyl)chromones from the agarwood of Aquilaria crassna in Laos. Fitoterapia 133:12–16.
- Konishi T, Iwagoe K, Kiyosawa S, Fujiwara Y. (1991). Studies on the agalwood (Jinko). XI. structure of dioxan-linked bi-phenylethylchromone derivative. Chem Pharm Bull 39:1869–1870.
- Huo HX, Zhu ZX, Song YL, Shi SP, Sun J, et al. (2018). Anti-inflammatory dimeric 2-(2-phenylethyl)chromones from the resinous wood of Aquilaria sinensis. J Nat Prod 81(3):543–553.
- Xia LL, Li W, Wang H, Chen H, Cai C, et al. (2019). LC-MS guided identification of dimeric 2-(2-phenylethyl)chromones and sesquiterpene-2-(2-phenylethyl)chromone conjugates from agarwood of Aquilaria crassna and their cytotoxicity. Fitoterapia 138:104349.
- Yang Y, Chen HQ, Kong FD, Zhou LM, Li W, et al. (2018). Dimeric sesquiterpenoid-4H-chromone derivatives from agarwood of Aquilaria crassna and their cytotoxicity. Phytochemistry 145:207–213.
- Konishi T, Iwagoe K, Kiyosawa S, Fujiwara Y. (1989). Tri-2-(2-phenylethyl)chromones from agalwood. Phytochemistry. 28(12):3548–3550.
- Meier M, Kohlenberg B, Braun N. (2003). Isolation of anisyl acetone from agarwood oil. J Essent Oil Res 15(1):54–56.
- Li W, Mei WL, Zuo WJ, et al. (2016). Chemical constituents of Chinese agarwood induced by artificial holing. J Trop Subtrop Bot 24:342–347.
- Liao G, Dong WH, Yang JL, Li W, Wang J, et al. (2018). Monitoring the chemical profile in agarwood formation within one year and speculating on the biosynthesis of 2-(2-phenylethyl)chromones. Molecules 2(6)3:1261.
- Lin BD, Zhou B, Dong L, Wu Y and Yue JM. (2016). Formosins A–F: diterpenoids with anti-microbial activities from Excoecaria formosana. Nat Prod Bioprospect 6(1):57–61.
- Wu HC, Cheng MJ, Yen CH, Chen YMA, Chen YS, et al. (2020). Chemical constituents with GNMT-promoter-enhancing and NRF2-reduction activities from Taiwan agarwood Excoecaria formosana. Molecules 25(7):1746.
- Sagris M, Theofilis P, Antonopoulos AS, Oikonomou E, Paschaliori C , et al. (2021). Inflammation in coronary microvascular dysfunction. Int J Mol Sci. 22(24):13471.
- Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Pereira CS, Teixeira AA, et al. (2021). Inflammation in human heart failure: major mediators and rherapeutic targets. Front Physiol 12:746494.
- Yadav DK, Mudgal V, Agrawal J, AK Maurya, DU Bawankul, et al. (2013). Molecular docking and ADME studies of natural compounds of agarwood oil for topical anti-inflammatory activity. Curr Comput Aided Drug Des 9(3):360–370.
- Zhu ZX, Gu YF, Zhao YF, Song Y, Li J, et al. (2016). GYF-17, a chloride substituted 2-(2-phenethyl)-chromone, suppresses LPS-induced inflammatory mediator production in RAW264.7 cells by inhibiting STAT1/3 and ERK1/2 signaling pathways. Int Immunopharmacol 35:185–192.
- Wang C, Peng D, Liu Y, Wu Y, Guo P, et al. (2021). Agarwood alcohol extract protects against gastric ulcer by inhibiting oxidation and inflammation. Evid Based Complement Alternat Med 2021:9944685.
- Okugawa H, Ueda R, Matsumoto K, Kawanishi K, and Kato A. (1993). Effects of agarwood extracts on the central nervous system in mice. Planta Med 59(1):32–36.
- Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato K. (2000). Effects of sesquiterpenoids from “oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2a receptors in rat brain. Phytomedicine 7(5):417–422.
- Okugawa H, Ueda R, Matsumoto K, Kawanishi K, and Kato A. (1996). Effect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Planta Med 62(1):2–6.
- Takemoto H, Ito M, Shiraki T, Yagura T, Honda G, et al. (2008). Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J Nat Med 62(1):41–46.
- Wang S, Zhou Y, Fuchao M, Zhang QY, Liu YY, et al. (2016). Effect of agarwood produced by whole-tree agarwood-inducing technique on hypnotic and spontaneous activity inhibition of mice. J Int Pharm Res 43:1082–1087.
- Wang S, Wang C, Peng D, Liu X, Wu C, et al. (2017). Agarwood essential oil displays sedative-hypnotic effects through the GABAergic system. Molecules 22(12):2190.
- Wang S, Wang C, Yu Z, Wu C, Peng D, et al. (2018). Agarwood essential oil ameliorates restrain stress-induced anxiety and depression by inhibiting HPA axis hyperactivity. Molecules 19(11):3468.
- Yang L, Qiao LR, Ji CX, Xie D, Gong NB, et al. (2013). Antidepressant abietane diterpenoids from Chinese eaglewood. J Nat Prod 76(2):216–222.
- Castro KP, Ito M. (2021). Individual and combined inhalational sedative effects in mice of low molecular weight aromatic compounds found in agarwood aroma. Molecules 26(5):1320.
- Kao WY, Hsiang CY, Ho SC, Ho TY and Lee KT. (2021). Novel serotonin-boosting effect of incense smoke from Kynam agarwood in mice: The involvement of multiple neuroactive pathways. J Ethnopharmacol 275:114069.
- Peng X, Fan R, Xie L, Shi X, Dong K, et al. (2022). A growing link between circadian rhythms, type 2 diabetes mellitus and alzheimer's disease. Int J Mol Sci 23(1):504.
- Liu Y, Wang S, Zhou Y, Zhang QY, Ma FC, et al. (2022). Effect of agarwood extracts produced by the whole-tree agarwood-inducing technique on gastrointestinal motility and gastric ulcer. J Int Pharm Res 43:1076–4081.
- Yang M, Li YF, Ma QS, et al. (2011). Effect of 50% ethanol-extract from bawei chenxiang powder on hemodynamics in myocardial ischemia rats. Chin J Trad Chin Med 11:2740–2742.
- Chunyan Y, ZhuYanmei WL, Qiong W. (2011). Effect of Tibetan bawei chenxiang powder on cerebral ischemia reperfusion injury in rats with apoptosis neurons at hippocampal CA1 area. J QH Med Colle 4:243–246.
- Wang C, Peng D, Liu Y, Yu Z, Guo P, et al. (2020). Agarwood alcohol extract ameliorates isoproterenol-induced myocardial ischemia by inhibiting oxidation and apoptosis. Cardiol Res Pract 3640815.
- Jermsri P, Jiraviriyakul A, Unajak S, Kumphune S. (2012). Effect of Aquilaria crassna crude extract on simulated ischemia induced cardiac cell death. Int J Pharm Bio Sci 3(3):604‒613.
- Kumphune S, Jermsri P, Paiyabhroma N. (2012). An in vitro anti-ischemic effect of ethyl acetate extract of Aquilaria crassna in isolated adult rat ventricular myocytes subjected to simulated ischemia. J. Phytother Pharmacol 5:47‒54.
- Suwannasing C, Paiyabhroma N, Kumphune S. (2012). Anti-ischemic effect of ethyl acetate extract of Aquilaria crassna by attenuation of p38-MAPK activation. J Appl Pharm Sci 2(10):26‒30.
- Jermsri P, Kumphune S. (2012). Ethyl acetate extract of Aquilaria crassna preserve actin cytoskeleton on simulated ischemia induced cardiac cell death. J Med Plant Res 6(23):4057‒4062.
- Matera MG, Ora J, Cavalli F, Rogliani P, Cazzola M. (2021). New avenues for phosphodiesterase inhibitors in asthma. J Exp Pharmacol 13:291‒302.
- Zhu ZX, Zhao YF, Huo HX, Gao X, Zheng J, et al. (2016). HHX-5, a derivative of sesquiterpene from Chinese agarwood, suppresses innate and adaptive immunity via inhibiting STAT signaling pathways. Eur J Pharmacol 791:412–413.
- Guo R, Li J, Gu YF, Li Y, Li S, et al. (2019). GYF-21, an epoxide 2‑(2‑phenethyl)‑chromone derivative, suppresses dysfunction of B cells mainly via inhibiting BAFF activated signaling pathways. Int Immunopharmacol 67:473–482.
- Wang C, Wang S, Peng D, Yu Z, Guo P, et al. (2019). Agarwood extract mitigates intestinal injury in fluorouracil-induced mice. Biol Pharm Bull 42(7):1112‒1119.
- Wang CH, Peng DQ, Liu YY, Wu YL, Guo P, et al. (2021). Anti-asthmatic effect of agarwood alcohol extract in mice and its mechanism. Zhongguo Zhong Yao Za Zhi 46(16):4214‒4221.
- Fu K, Xu M, Zhou Y, Li X, Wang Z, et al. (2020). The status quo and way forwards on the development of Tibetan medicine and the pharmacological research of Tibetan materia medica. Pham Res 155:104688.
- Gao AR. (2018). Clinical observation of BaWwei Chenxiang powder in the treatment of coronary heart disease and angina pectoris. Latest Med Inf Abs 18:246–254.
- Wang YQ, Liu F. (2017). Clinical therapeutic effects on functional constipation treated with the external application of Chenxiang Tongbian powder on the Umbilicus and Moxibustion. World J Integr Tradit West Med 12:1546–1549.
- Zakaria F, Talip BA, Kahar EEM, Muhammad N, Abdullah N, et al. (2020). Solvent used in extraction process of agarwood: a systematic review. Food Res 4(3):731–737.
- Liu YY, Wei JH, Gao ZH, Zhang Z, Lyu JC. (2017). A review of quality assessment and grading for agarwood. Chin Herb Med 9(1):22–30.
- Gao X, Xie M, Liu S, Guo X, Chen X, et al. (2014). Chromatographic fingerprint analysis of metabolites in natural and artificial agarwood using gas chromatography-mass spectrometry combined with chemometric methods. J Chromatogr B 967:264–273.
- Hashim Y, Ismail N, Abbas P. (2014). Analysis of chemical compounds of agarwood oil from different species by gas chromatography mass spectrometry (GCMS). IIUM Engineering J 15(1)55–60.
- Yoneda K, Yamagata E, Nakanishi T, Nagashima T, Kawasaki I, et al. (1984). Sesquiterpenoids in two different kinds of agarwood. Phytochemistry 3(21):2068–2069.
- Ishihara M, Tsuneya T, Uneyama K. (1993). Components of the volatile concentrate of agarwood. J Essent Oil Res 5:283–289.
- Tamuli P, Boruah P, Nath SC, Leclercq P. (2005). Essential oil of eaglewood tree: A product of pathogenesis. J Essent Oil Res 17:601–604.
- Bhuiyan MNI, Begum J, Bhuiyan MNH. (2008). Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. Bangladesh J Pharmacol 4(1):24–28.
- Pripdeevech P, Khummueng W, Park SK. (2011). Identification of odor-active components of agarwood essential oils from Thailand by solid phase microextraction-GC/MS and GC-O. J Essent Oil Res 23(4):46–53.
- Yoswathana N. (2013). Extraction of agarwood (Aquilaria crassna) oil by using supercritical carbon dioxide extraction and enzyme pretreatment on hydrodistillation. J Food Agric Environ 11(2):1055–1059.
- Jong PL, Pascale T, Rozi M. (2014). Gas chromatography-mass spectrometry analysis of agarwood extracts from mature and juvenile Aquilaria malaccensis. Int J Agric Biol 16(3):644–648.
- Mei WL, Yang DL, Wang H, Yang JL, Zeng YB, et al. (2013). Characterization and determination of 2-(2-phenylethyl)chromones in agarwood by GC-MS. Molecules 18(10):12324–12345.
- Shimada Y, Tominaga T, Kiyosawa S. (1986). Studies on the agalwood.(Jinko). IV: correlation between the grading of agalwood on the market and the chromone derivatives. Yakugaku Zasshi J Pharm Soc Jpn 106(5):391–397.
- Yu M, Liu Y, Feng J, Chen D, Yang Y, et al. (2021). Remarkable phytochemical characteristics of chi-nan agarwood induced from new-found chi-nan germplasm of Aquilaria sinensis compared with ordinary agarwood. Int J Anal Chem 2021:5593730.
- Xie B, Luo H, Huang X, Huang F, Zhang Q, et al. (2021). Pharmacokinetic studies of six major 2-(2-phenylethyl) chromones in rat plasma using ultra high performance liquid chromatography with tandem mass spectrometry after oral administration of agarwood ethanol extract. J Sep Sci 44(12):2418‒2426.
- Chen Y, Shang L, Yang J, Yun LG, Fang YY, et al. (2017). The exploration of identification method of wild agarwood. Scientia Silvae Sinicae 53(9):90‒96.
- Shang L, Chen Y, Yan T, Zou X, Li G, et al. (2018). HPLC characteristic chromatogram of agarwood. Scientia Silvae Sinicae 54(7):104‒111.
- Chen X, Liu Y, Yang Y, Jian Feng, Peiwei Liu, et al. (2018). Trunk surface agarwood-inducing technique with Rigidoporus vinctus: an efficient novel method for agarwood production. PLoS One 13(6):e0198111.
- Yang JL, Dong WH, Kong FD, Liao G, Wang J, et al. (2016). Characterization and analysis of 2-(2-phenylethyl)-chromone derivatives from agarwood (Aquilaria crassna) by artificial holing for different times. Molecules 21(7):911.
- Yang J, Dong W, Chen H, Kong F, Wang J, et al. (2018). Qualitative and quantitative analysis of flidersiachromones in three agarwood samples by HPLC-MS/MS. Chem Res Chin Univ 34:389–396.
- Gao M, Han X, Huang J, Sun Y, Liu Y, et al. (2021). Simultaneous determination of multiple active 2-(2-phenylethyl)chromone analogues in agarwood by HPLC, QAMS, and UPLC-MS. Phytochem Anal 32(3):412‒422.
- Takamatsu S, Ito M. (2020). Agarotetrol in agarwood: its use in evaluation of agarwood quality. J Nat Med 74(1):98‒105.
- Feng J, Liu Y, Wei J. (2017). Detection of rosin acids in 44 batches of domestic agarwood medicinal materials from different sources. China Modern Chinese Med 19(8):1071‒1075.
- Antonopoulou M, Compton J, Perry LS, Mubarak RA. (2010). The trade and use of agarwood (Oudh) in the United Arab Emirates, TRAFFIC Southeast Asia, Petaling Jaya, Selangor, Malaysia. 2010.
- Lancaster C, Espinoza E. (2012). Evaluating agarwood products for 2-(2-phenylethyl)chromones using direct analysis in real time time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 26(23):2649–2656.
- Espinoza EO, Lancaster C, Kreitals NM, Hata M, Cody RB, et al. (2014). Distinguishing wild from cultivated agarwood (Aquilaria spp.) using direct analysis in real time and time of-flight mass spectrometry. Rapid Commun. Mass Spectrom 28(3):281–289.
- Yao C, Qi L, Zhong F, Li N and Y Ma. (2021). An integrated chemical characterization based on FT-NIR, GC-MS and LC-MS for the comparative metabolite profiling of wild and cultivated agarwood. J Chromatogr B 1188:123056.
- Tian C, Wu A, Yao C, Song Z, Shen L, et al. (2021). UHPLC-QTOF-MS based metabolite profiling analysis and the correlation with biological properties of wild and artificial agarwood. J Pharm Biomed Anal 194:113782.
- Xie Y, Li L, Chen Y, Yang Y, Xu H, et al. (2020). Rapid authentication of agarwood by using liquid extraction surface analysis mass spectrometry (LESA-MS). Phytochem Anal 31(6):801‒808.
- Chen Y, Yan T, Zhang Y, Wang Q, Li G, et al. (2020). Characterization of the incense ingredients of cultivated grafting Kynam by TG-FTIR and HS-GC-MS. Fitoterapia 142:104493.
- Du TY, Dao CJ, Mapook A, Stephenson SL, Elgorban AM, et al. (2022). Diversity and Biosynthetic Activities of Agarwood As
 Abstract
Abstract  PDF
PDF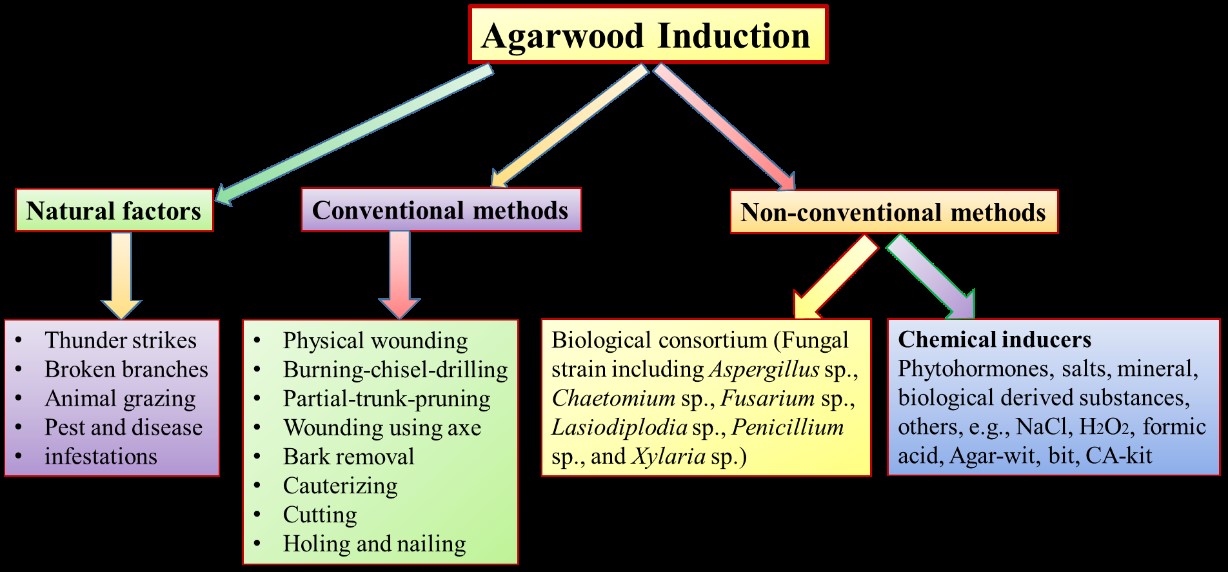
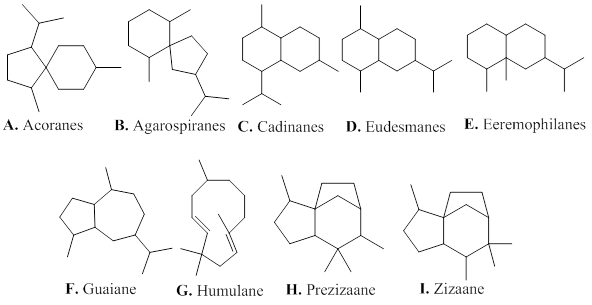
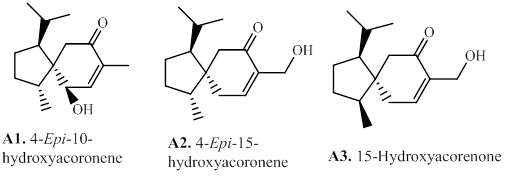
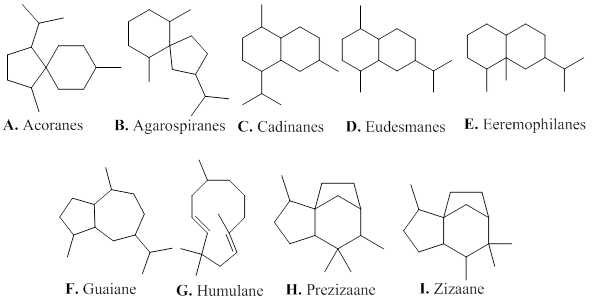
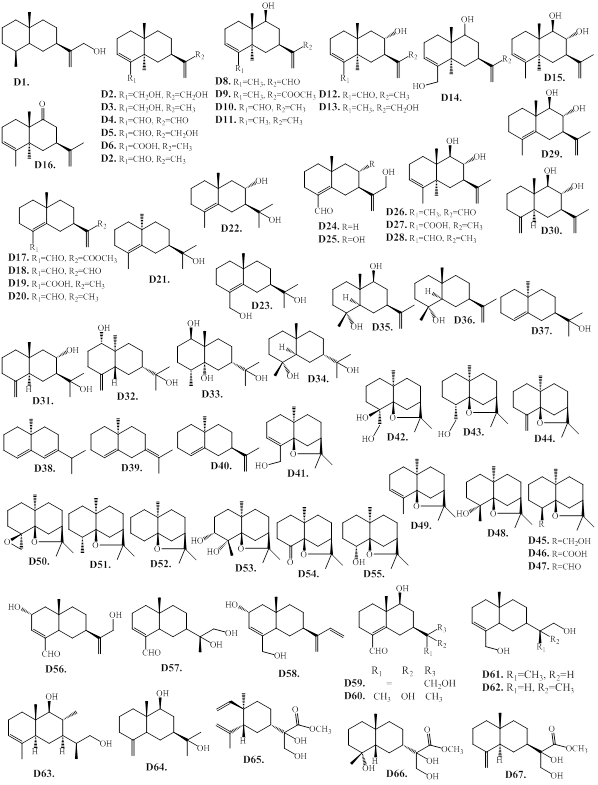
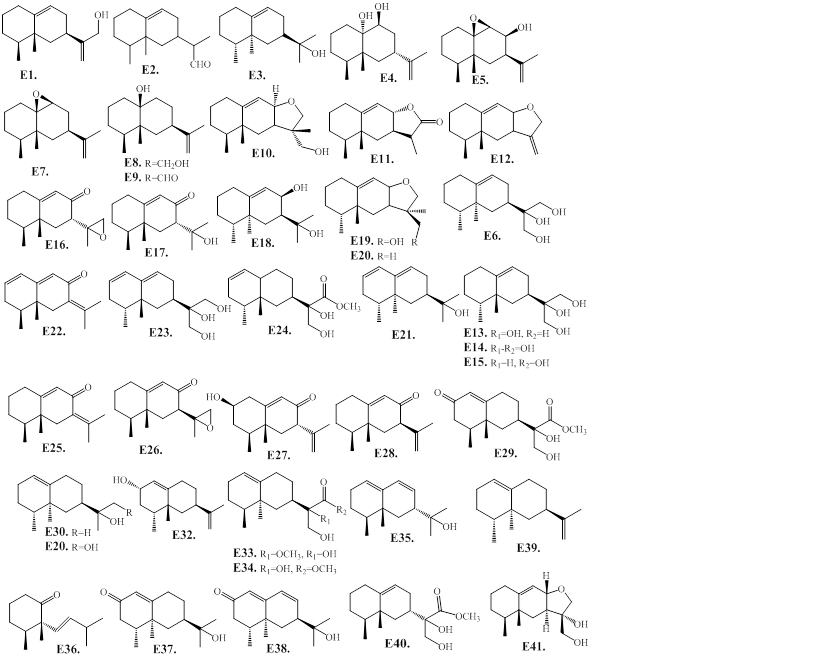
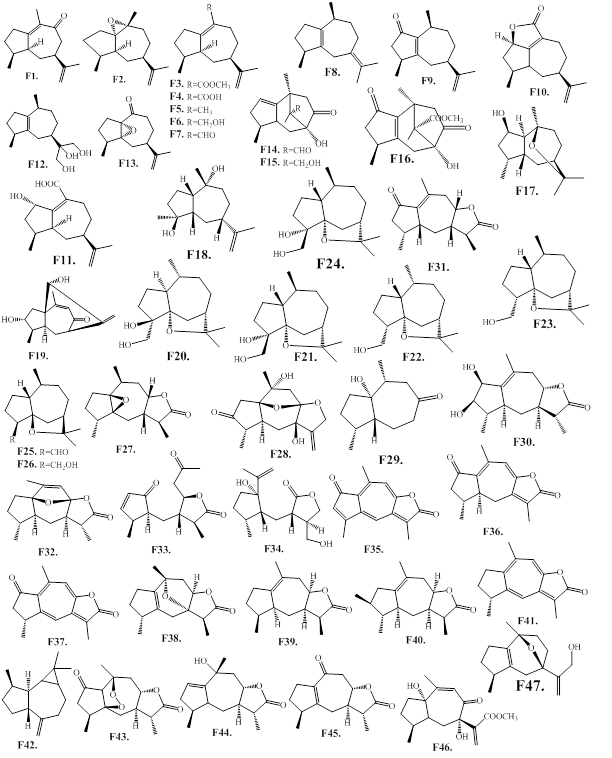
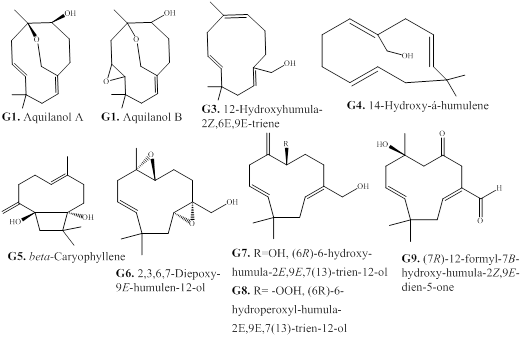
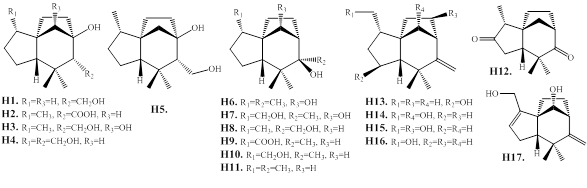
.png)