Past Issues
Improvement of Self-Perceived Tinnitus and Speech Discrimination in Noise by Lyophilized Powder of Enzymolyzed Honeybee (Apis melifera) Larvae in Older Adults with Age-Related Hearing Loss: A Double-Blind Placebo-Controlled, Randomized Study
Mitsuhiro Aoki1,2*, Hiroshi Okuda1, Natsuko Obara1
1 Department of Otolaryngology, Gifu University Graduate School of Medicine, Japan
2 Department of Otolaryngology, Center for dizziness and hearing loss, Ogaki Tokushukai Hospital, Japan
*Corresponding author: Mitsuhiro Aoki, Mitsuhiro Aoki, MD, Ph.D., 6-85-1 Hayashi-machi, Ogaki City, 503-0015 Gifu, Japan; Email: [email protected]
Citation: Aoki M, et al. (2022). Improvement of Self-Perceived Tinnitus and Speech Discrimination in Noise by Lyophilized Powder of Enzymolyzed Honeybee (Apis melifera) Larvae in Older Adults with Age-Related Hearing Loss: A Double-Blind Placebo-Controlled, Randomized Study. Traditional Medicine. 3(2):11.
Received Date: Aug 30, 2022
Published Date: Sep 19, 2022
Copyright: Aoki M© (2022).
ABSTRACT
Background: Tinnitus troubles many older adults accompanying an increased threshold of high-frequency hearing, and most older adults struggle to understand conversations in noise. Objectives: An exciting power of honeybee products has been reported as alternative medicine. We also previously reported that lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae may have a therapeutic effect on hearing loss or tinnitus. Methods: Fifty-eight older adults with age-related hearing loss were recruited from nearby Japan regions through online advertisements. Participants were randomized (1:1) to either honeybee (Apis melifera) larvae or placebo interventions for 12 weeks for a double-blind placebo-controlled, randomized study. The outcome was evaluated with pure-tone hearing levels, a visual analog scale (VAS) to rate tinnitus severity, and speech discrimination in quiet and noisy conditions. The effects of the honeybee (Apis melifera) larvae on the neurosteroid related to the hypothalamic-pituitary-adrenal (HPA) axis (cortisol, dehydroepiandrosterone sulfate (DHEAS)) and inflammatory cytokines (IL-2R, IL-6 and TNFα) were also estimated. Results: The honeybee (Apis melifera) larvae significantly improved the speech discrimination in noise and reduced the VAS of tinnitus loudness despite showing no effects on the hearing level and speech discrimination in quiet conditions. The intervention of the honeybee (Apis melifera) larvae was related to the reduction of the cortisol/DHEAS ratio. Conclusions: Our results suggest that the honeybee (Apis melifera) larvae have the potential to improve impaired speech discrimination in noise and self-perceived tinnitus for older adults with age-related hearing loss by modulating the endocrine pathways of the HPA system associated with auditory cognitive function-related centers.
Keywords: honeybee (Apis melifera) larvae; age-related hearing loss; tinnitus; dehydroepiandrosterone sulfate; speech discrimination
INTRODUCTION
Tinnitus troubles many older adults, accompanying an increased threshold of high-frequency hearing. Approximately 30 % of the older adult population perceive tinnitus, and its prevalence increases with age. Age-related hearing loss is associated with cognitive impairment and contributes to the development of depression. The decline of speech understanding ability with aging disturbs social communication and exacerbates tinnitus-related depression [1, 2]. Furthermore, even with mild hearing loss in pure tone audiometry, most older adults feel difficult to understand conversations in noise. However, the factors contributing to the decline of speech discrimination in noise in older adults remain unclear [3]. Although an urgent need in clinical practices has been required to treat age-related hearing loss and poor speech discrimination with aging, no cure has ever been found.
We previously reported that administering lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae to tinnitus sufferers relieved depressive symptoms associated with tinnitus and decreased hearing thresholds of 2000 and 4000Hz in pure tone audiogram [4]. The honeybee (Apis melifera) larvae are rich in amino acids, which become the maximum level on the 21st day after breeding and decrease with growing. Mature drone larvae on the 18~21st day after breeding are the preferred choice for the honeybee (Apis melifera) larvae. The drone larvae have been prepared for human consumption in Asian countries for an extended period because the drone larvae are much bigger than the worker larvae. The lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae has been commercially available as a nutritional supplement in Japan, and it is rich in vitamins, amino acids, fatty acids, and carbohydrates, especially glycogen [5].
Our previous study demonstrated that the honeybee (Apis melifera) larvae have the potential to normalize a function of the hypothalamic-pituitary-adrenal (HPA) axis by reducing the cortisol to dehydroepiandrosterone sulfate (DHEAS) ratio (C/D ratio), which is reported to be higher in depressed subjects than in normal subjects [6]. We previously reported that the the lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae improve pure tone hearing levels in quiet condition. We concluded that the lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae could save chronic self-perceived tinnitus, and it potentially has a high immunomodulatory activity for auditory pathways related to tinnitus. However, we could not investigate whether the honeybee larvae can improve speech discrimination, especially in noise.
Recent reports demonstrated that chronic inflammation might be contributed to age-related hearing loss and poor speech discrimination in older adults [7, 8]. Based on the previous report associated with age-related diseases, inflammaging may be an essential component in the pathophysiology of presbycusis, which may be caused by low-grade inflammation processes [7, 9]. Also, Inflammatory cytokines such as TNF-α, released from monocytes, macrophages, microglia and astrocytes activate the HPA axis by modulating the central mono-amine functions [10]. An overproduction of TNF-α in the auditory cortex produces tinnitus through chronic neuroinflammation [11]. Furthermore, the DHEAS may reduce chronic inflammation [12]. This study is a double-blind placebo-controlled, randomized trial to clarify the effect of the lyophilized powder of enzymolized honeybee (Apis melifera) larvae on hearing levels, speech discrimination in quiet or noise, and self-perceived tinnitus in older adults with mild or moderate hearing loss associated with aging.
METHODS
This study was approved by the local ethic committee of Gifu University Graduate School of Medicine and registered in the University Hospital Medical Information Network Clinical Trials Registry in Japan (UMIN 34706). All participants in this study who were more than 60 years old and aware of hearing loss with self-perceived tinnitus were recruited from the nearby region in Japan through online advertisements. Also, they met the following selection criteria and never met any exclusion criteria. Informed consent was obtained by writing from all participants.
We confirmed no perforations of the eardrums on both sides as selected criteria. All participants had mild sensorineural hearing loss (26 to 40 dB by the average of 500, 1000, 2000, and 4000Hz) or moderate sensorineural hearing loss (41 to 60 dB by the average of 500, 1000, 2000, and 4000Hz) in the pure tone audiometry as the screening test of both ears. They were aware of tinnitus on one or both sides when obtaining consent.
Subjects were also excluded from this study if they regularly drank healthy foods and supplements containing bee cubs and treated acute otitis media and acute deafness (within six months after the onset of acute deafness) after obtaining consent. We also excluded subjects with a clinical history of Ménière's disease, brain tumor, genetic deafness, cerebrovascular accidents, and cranial nerve diseases.
They replied to a Hearing Handicap Inventory for Elderly (HHIE) questionnaire, used in clinical practice to quantify the impact of hearing handicaps on daily living [13]. The each of the 10-question items was evaluated on a 3-point scale. (Yes = 4, Sometimes = 2, and No = 0).
The severity of their tinnitus (loudness, duration and annoyance) on a Visual Analog Scale (VAS) was assessed by indicating the location on a 100-mm line (not at all – very severe). The VAS was determined by measuring in mm from the left side. The average pre-intervention VAS was calculated as the log of daily VAS for one week before the intervention.
All participants received the pure-tone audiometry in a quiet room before and after the 12-week intervention, and the average hearing level of 0.5, 1, 2 and 4 kHz was evaluated. Two speakers were placed 30 degrees to the left and right in front of the subject to test speech discrimination. From one side speaker, 20 Japanese syllables were presented at 65dB each and no sound was present from another side speaker, and the percentage of correct answers was used as speech discrimination (speech discrimination in quiet). We also measured the speech discrimination under the condition that 55 dB-speech noise flowed from the other side speaker (speech discrimination in noise).
The study was designed as a single-center, randomized, double-blind, placebo-controlled, 12-week study. All participants who met the above-mentioned selected criteria in this study were randomized into two groups using a single list of interventions with either lyophilized powder of enzymolized honeybee (Apis melifera) larvae (720mg/4 capsules/day) or placebos (hydrogenated dextrin; 720mg/4 capsules/day) by before enrolling in this study. The grouping was performed so that there was no bias in sex, age and hearing level.
The lyophilized powder of honeybee (Apis melifera) larvae (20-21 days old drone) was obtained by degradation of honeybee larvae protein using protease and freeze-drying. The enzyme treatment and encapsulation were performed by Yamada Bee Company, Inc. (Okayama, Japan). The nutritional composition and chemical elements of the honeybee larvae (Apis melifera) are shown in Table 1.
Table 1: Nutritional composition and chemical elements of honeybee (Apis melifera) larvae.
|
Nutritional composition |
Chemical elements |
||||||
|
Water |
6.7 |
Sodium (Na) |
21.4 |
Thiamine (B1) |
1.88 |
||
|
Protein |
48.5 |
Calcium (Ca) |
49.3 |
Riboflavin (B2) |
2.96 |
||
|
Lipid |
20.8 |
Potassium |
1240 |
Niacin (B3) |
32.3 |
||
|
Minetral |
3.8 |
Magnesium |
92.1 |
Pyridoxine (B6) |
0.55 |
||
|
Glucose |
19.4 |
Phosphorus (P) |
813 |
Pantothenic acid (B5) |
4.35 |
||
|
Fiber |
0.48 |
Iron (Fe) |
6.42 |
Biotin (B7) |
0.05 |
||
|
Saturated fatty acid |
10.6 |
Copper (Cu) |
1.67 |
Ascorbic acid (C) |
14 |
||
|
Monounsaturated fatty acid |
9.58 |
Zinc (Zn) |
7.33 |
Tocopherol (E) |
0.3 |
||
|
Polyunsaturated fatty acid |
0.28 |
Chromium (Cr) |
0.05 |
Choline |
620 |
||
|
Cholesterol |
0.002 |
Manganese (Mn) |
0.029 |
|
|
|
|
|
Data in g/100g of honeybee larvae |
Data in g/100g of honeybee larvae |
||||||
We collected blood sample from all participants before and after the 12-week intervention and examined biochemical items, inflammation-related cytokines, oxidative stress-related marker, and HPA axis related hormones, which includes Cortisol and DHEAS, and the C/D ratio, were also estimated. All participants answered the HHIE questionnaires before and after the 12-week interventions. The average post-intervention VAS score was calculated as the log of daily VAS from the 78th to the 84th day after the intervention.
Mann-Whitney U-test assessed the difference in collected data between both groups before and after the 12-week intervention. The differences in outcomes between before and after the intervention were analyzed using Wilcoxon signed-rank test. The p < 0.05 was considered significant, and power analysis was calculated for the Wilcoxon signed-rank test with a 5% significant level. The values in the text are presented as the means and one standard deviation, and the values in the Tables are shown as means (95% confidence intervals).
RESULTS
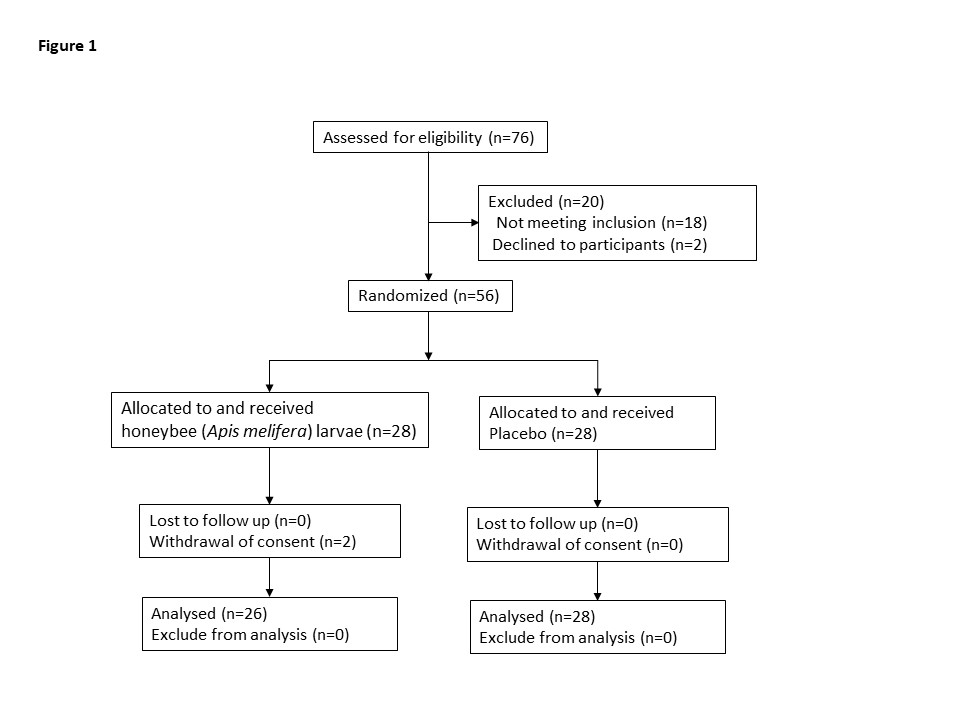
Of seventy-six applicants for this study, twenty were excluded, and overall, fifty-six applicants were randomly assigned to the interventions. Two subjects dropped out of this study to withdraw their consent. Consequently, fifty-four subjects, twenty-six received the lyophilized powder of enzymolyzed honeybee (Apis melifera) larvae (honeybee group), and twenty-eight received the placebo (placebo group), completed the trial (Figure 1). No side effects were reported in both groups during this study.
Figure 1: Flow diagram of the randomized, placebo-controlled study into the therapeutic effects of the honeybee (Apis melifera) larvae for older adults with age-related hearing loss.
No significant differences between the honeybee and placebo groups before the intervention were found in age (71.4±5.2 years old vs. 71.6±6.9 years old), sex ratio (female: male, 7:19 vs. 8:20), height (164.7±8.8cm vs. 161.2±6.9 cm), body weight (63.2±13.5kg vs. 63.2±14.0kg), blood pressure (systolic blood pressure/diastolic blood pressure; 128.3±14.1/77.1±13.0 mmHg vs. 131.2±18.6/79.9±11.2 mmHg) and prevalence of comorbidity (p > 0.05, Table 2).
Average hearing levels of the honeybee group (right ear: 39.2±10.0dB, left ear: 43.8±13.3dB) were not significantly different from those of the placebo group (right ear: 40.3±14.1dB, left ear: 43.1±8.9dB) before the intervention (p > 0.05, Table 2).
No significant difference in the average speech discrimination in quiet and noise before the interventions was found between both groups (p > 0.05, Table 2). Also, there was no significant differences in the VAS (loudness, duration, annoyance) between the honeybee group (30.7±23.9mm, 61.5±33.2mm, 33.2±22.9mm) and the placebo group (33.2±26.2mm, 48.3±39.8mm, 28.3±27.3mm, p > 0.05, Table 2) before the intervention. No significant difference in the average score of HHIE between the honeybee and placebo groups was found before the intervention (21.8±17.8 vs. 21.9±17.9, p > 0.05, Table 2).
|
|
Honeybee group |
Placebo group |
|
Age |
71.4 (69.0-73.8) |
71.6 (69.1-74.2) |
|
N |
26 |
28 |
|
Sex (Female:Male) |
7:19 |
8:20 |
|
Comorbidity |
|
|
|
Diabetes |
2 |
3 |
|
Hypertension |
6 |
5 |
|
Hyperlipidemia |
4 |
5 |
|
other |
3 |
3 |
|
HHIE |
21.81 (14.13-29.48) |
21.86 (15.22-28.5) |
|
VAS for tinnitus (mm) |
|
|
|
Loudness |
40.73 (31.56-49.90) |
33.18 (23.47-42.89) |
|
Duration |
61.50 (49.09-73.91) |
48.32 (33.58-63.06) |
|
Annoyance |
33.15 (24.30-42.01) |
28.32 (18.22-38.42) |
|
Pure-tone average hearing level (dB) |
|
|
|
Right |
39.17 (35.47-42.86) |
40.34 (36.45-44.24) |
|
Left |
43.78 (38.49-49.07) |
43.08 (38.54-47.61) |
|
Speech Discrimination (%) |
|
|
|
in quiet |
79.42 (69.02-89.83) |
80.00 (74.04-85.96) |
|
in noise |
58.27 (49.14-67.40) |
64.29 (57.60-70.97) |
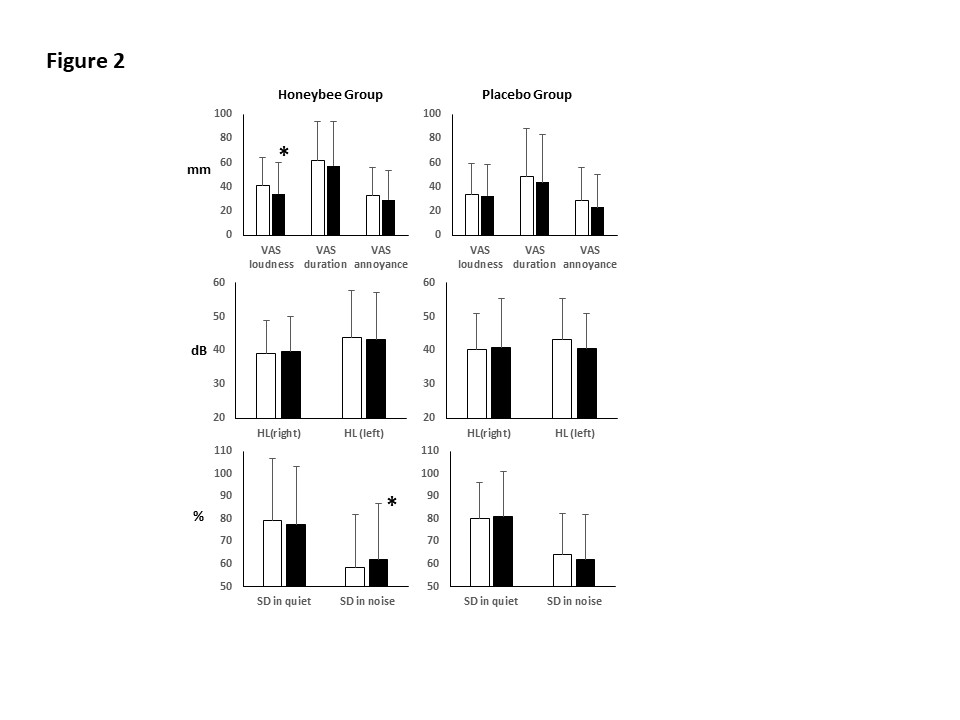
The intervention of the honeybee (Apis melifera) larvae significantly reduced the VAS for the tinnitus loudness, and the beneficial effect was more significant in subjects with mild hearing loss (p < 0.05, Figure 2), while there was no significant change in the VAS for tinnitus duration, annoyance, or the HHIE score by the intervention of the honeybee (Apis melifera) larvae (p > 0.05, Figure 2). No significant differences in the VAS between both groups at 12 weeks were found (p > 0.05, Figure 2).
Figure 2: Effects of honeybee (Apis melifera) larvae on the VAS for tinnitus, hearing levels and speech discrimination. White column: pre-intervention, Black column: post-intervention. *: significant different from pre-intervention at p < 0.05.
No significant change by the intervention in the average hearing levels was found in both groups (p > 0.05, Figure 2). The speech discrimination in quiet never changed during the intervention in both groups (p > 0.05, Figure 2). The 12-week intervention of the honeybee (Apis melifera) larvae significantly improved the speech discrimination in noise (p < 0.05, Figure 2), whereas no significant improvement was found in the placebo group (p > 0.05, Figure 2). No significant difference in hearing levels and speech discrimination in quiet and in noise between both groups at 12 weeks were found (p > 0.05).
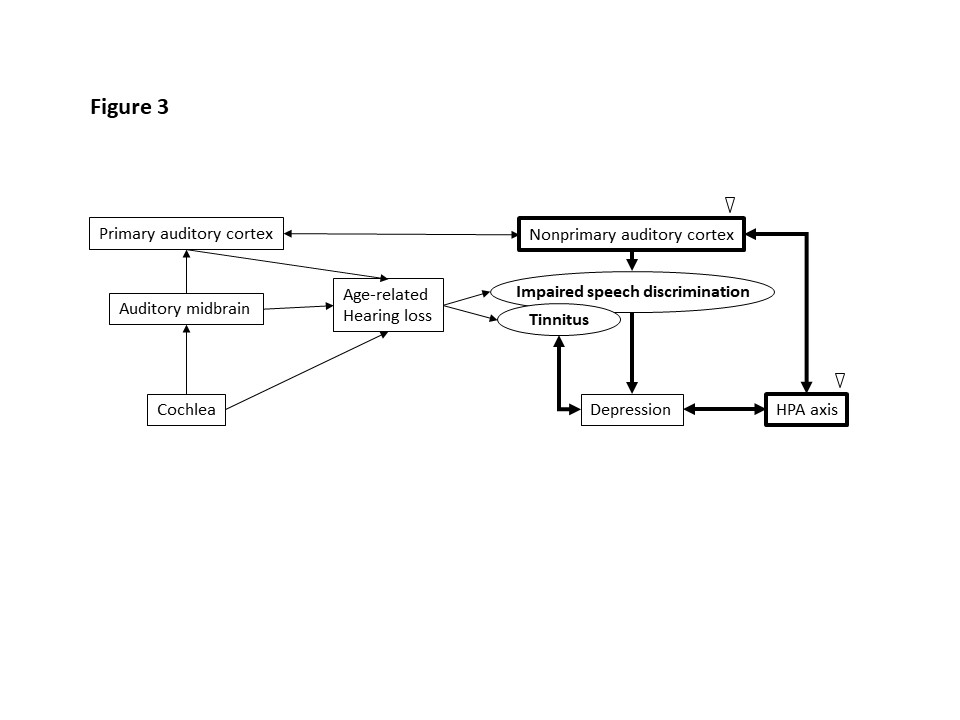
Figure 3: The effect of honeybee (Apis melifera) larvae on the pathway associated with impaired speech discrimination and tinnitus in older adults with age-related hearing loss. White arrowhead: possible working points of the honeybee (Apis melifera) larvae.
The 12-week intervention of honeybee (Apis melifera) larvae significantly decreased the C/D ratio (p < 0.05, Table 3). In contrast, the average level of DHEAS significantly decreased at 12 weeks in the placebo group (p < 0.05, Table 3). In other findings, the serum creatinine significantly increased by the honeybee (Apis melifera) larvae intervention and the HDL significantly decreased by the placebo intervention (p < 0.05, Table 3). There was no significant change during the 12-week intervention in other blood findings, including inflammatory parameters; CRP, IL-2R, IL-6, and TNFα (p > 0.05, Table 3). In addition, no significant difference between groups at 12 weeks was found in any blood findings (p > 0.05, Table 3). No effect of the honeybee (Apis melifera) larvae on the MDA-LDL was found in this study. However, the serum level of the MDA-LDL in these participants was higher than the normal range in the population aged more than 55 years old (61-105 U/L).
Table 3: Effects of honeybee (Apis melifera) larvae on the blood findings.
|
Honeybee Group |
Placebo Group |
||||||
|
|
Pre-intervention |
Post-intervention |
p-value |
Pre-intervention |
Post-intervention |
p-value |
|
|
CRP (mg/dL) |
0.09 (0.06-0.13) |
0.08 (0.05-0.11) |
0.16 |
0.16 (0.07-0.25) |
0.12 (0.04-0.19) |
0.22 |
|
|
IL-2R (U/mL) |
319.88 (284.86-354.91) |
318.54 (292.34-344.74) |
0.45 |
366.36 (327.42-405.29) |
325.46 (216.11-434.82) |
0.23 |
|
|
IL-6 (pg/mL) |
2.06 (1.13-2.98) |
1.89 (1.34-2.43) |
0.33 |
2.29 (1.55-3.03) |
2.29 (1.20-3.38) |
0.49 |
|
|
HDL (mg/mL) |
59.23 (52.61-65.85) |
59.31 (53.53-65.08) |
0.48 |
60.14 (55.05-65.24) |
57.79 (52.97-62.60) |
0.01 |
|
|
LDL (mg/dL) |
112.69 (101.08-124.30) |
115.38 (102.35-128.42) |
0.24 |
115.18 (104.23-126.13) |
112.18 (100.62-123.74) |
0.08 |
|
|
MDA-LDL (U/L) |
103.19 (84.18-122.20) |
104.38 (84.53-124.24) |
0.43 |
99.61 (88.22-110.99) |
91.57 (83.29-99.8) |
0.06 |
|
|
DHEAS (μg/dL) |
102.70 (81.23-124.17) |
105.67 (82.87-128.47) |
0.22 |
90.14 (72.93-107.36) |
82.29 (67.59-96.98) |
0.01 |
|
|
HbA1c (%) |
5.88 (5.54-6.22) |
5.88 (5.49-6.25) |
0.32 |
5.89 (5.65-6.13) |
5.78 (5.57-6.00) |
0.06 |
|
|
UA (mg/dL) |
5.52 (4.95-6.10) |
5.53 (4.95-6.12) |
0.23 |
5.33 (4.81-5.86) |
5.26 (4.75-5.77) |
0.35 |
|
|
Cre (mg/dL) |
0.78 (0.71-0.85) |
0.81 (0.74-0.88) |
0.03 |
0.81 (0.70-0.92) |
0.84 (0.75-0.92) |
0.23 |
|
|
Cortisol (μg/dL) |
8.85 (7.90-9.79) |
7.91 (6.89-8.93) |
0.15 |
8.13 (0.66-9.59) |
8.07 (7.10-9.03) |
0.47 |
|
|
Cortisol/DHEAS |
0.12 (0.09-0.14) |
0.09 (0.08-0.11) |
0.04 |
0.13 (0.10-0.15) |
0.12 (0.10-0.14) |
0.48 |
|
|
Alb (g/dL) |
4.17 (4.03-4.30) |
4.14 (4.02-4.26) |
0.12 |
4.22 (4.11-4.32) |
4.15 (4.05-4.26) |
0.09 |
|
|
BUN (mg/d) |
18.23 (16.41-20.04) |
18.15 (16.69-19.60) |
0.34 |
16.44 (14.78-18.10) |
16.80 (15.09-18.52) |
0.12 |
|
|
AST(U/L) |
25.45 (20.34-30.56) |
24.42 (20.21-28.54) |
0.47 |
24.35 (21.17-27.52) |
22.61 (20.14-25.08) |
0.20 |
|
|
ALT(U/dL) |
20.10 (16.14-24.06) |
21.04 (16.68-25.39) |
0.07 |
20.50 (17.82-23.18) |
19.82 (15.83-23.81) |
0.45 |
|
|
TNFα(pg/mL) |
0.77 (0.70-0.83) |
0.76 (0.68-0.85) |
0.44 |
|
0.74 (0.66-0.83) |
0.74 (0.64-0.84) |
0.26 |
CRP: C-reactive protein, IL-2R: soluble IL-2 receptor, IL-6: Interleukin-6, TNFα: tumor necrosis factor α, HDL: high-density lipoprotein cholesterol, LDL: low-density lipoprotein cholesterol, MDA-LDL: malondialdehyde-modified low-density lipoprotein, DHEAS: dehydroepiandrosterone sulfate, HbA1c: hemoglobin A1c, UA: uric acid, Cre: creatinine, Alb: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
DISCUSSION
Our study showed that 12-week intervention of the lyophilized powder of enzymolized honeybee (Apis melifera) larvae significantly improved the speech discrimination in noise and no significant effect of the placebo on the speech discrimination was found. No effect on the hearing threshold in the pure tone hearing test or speech discrimination in quiet was found. There was a stronger tendency to improve the speech discrimination by the honeybee (Apis melifera) larvae in older adults with mild hearing loss rather than in older adults with moderate hearing loss. The VAS on the magnitude of self-perceived tinnitus was significantly reduced in the honeybee larvae group. Also, the C / D ratio was significantly decreased by the 12-week intervention of the honeybee (Apis melifera) larvae. On the other hand, the DHEAS was decreased in the placebo group. The present study demonstrated that the lyophilized powder of enzymolized honeybee (Apis melifera) larvae may be contributed to older adults suffering from hearing loss or self-perceived tinnitus and may reduce hyperactivity of the HPA system in age-related diseases. However, it is unclear how the honeybee larvae act on older adults with hearing impairment and tinnitus.
Sound signals encoded by the peripheral auditory system are conveyed to the auditory cortex via the cochlear nucleus, superior olive nucleus, lateral lemniscus, inferior colliculus, and medial geniculate nucleus and are cognitively processed by various central processes. It is not always necessary to undergo various central processing in pure tone audiometry. The speech signals are transmitted to the center by converting the information of multiple sound sources into one cochlear nerve. The complicated spike information is unraveled by extracting non-important signals and recognized in the central auditory system. Especially, understanding speech in noise requires extracting the necessary signal from the spike information. Selective attention to the critical signal and its continuation and ignoring unnecessary information may be essential to recognize required auditory information [14, 15].
The cortical activation of the nonprimary auditory cortex helps to understand speech in noise [16] by suppressing the encoded noise signals in neural responses via Heschl's Gyrus, the Planum Temporale (PT), and the Superior Temporal Gyrus [17]. Electrical stimulation of the PT sites engages inhibitory connection and consequently enhances the removal of noise from the speech and environmental sounds [18]. It is, therefore, highly possible that the enhancement of neural activity in the nonprimary auditory area may improve the speech discrimination in noise even in older adults with presbycusis.
The decline of speech discrimination in noise with aging may be explained by various hypothetical mechanisms [3, 19, 20]. One is a decline in peripheral cochlear function. Beyond sixty, the hearing threshold rises, making consonant-level speech worse. The second is central auditory disorders, the so-called dysfunction of the cochlear nerve's central pathways. However, little is known about the involvement of central auditory disorders associated with presbycusis. Finally, there is a difficulty in language comprehension associated with cognitive decline. The factors associated with peripheral cochlear dysfunction are the most common, and the involvement of the central auditory system and cognitive dysfunction in the age-related decline of speech discrimination is controversial [3].
As opposed to listening in quiet, listening in noise increases activation in the auditory cortex, especially in brain regions including the upper right temporal gyrus. Some reports evidenced that cognitive and perceptual training incorporating high cognitive demands can improve speech discrimination in noise by activating the subcortical cortex involved in memory and attention. However, little is known about subcortical and cortical processes in the cognitive training.
The cognitive impairment has been associated with reduced levels of the neurosteroid DHEA with aging [21, 22]. The metabolite of DHEA, DHEAS, which has a long biological half-life and stable serum levels, acts as a non-competitive antagonist on GABA-A receptors in the brain. The DHEAS may function as a positive allosteric modulator of NMDA receptors. The NMDA receptors on the membrane of the spine in dendrites play essential roles in establishing short-term memory, and older women with high DHEA levels in the serum have a better working memory, attention, and fluency in speech than those with low DHEA levels [23, 24].
Hippocampal atrophy may be accelerated by age-related diminished visual, auditory, tactile, and olfactory sensory input stimuli. Some studies using patients with depression showed that hippocampal atrophy was significantly correlated with the C/D ratio [6, 25]. Our previous study reported that older adults with moderate or severe hearing impairment showed a significantly higher C/D ratio and hippocampal atrophy % than older adults with normal hearing levels or mild hearing impairment [26].
Overproduction of cortisol may damage the inner ear because many glucocorticoid receptors exist in inner ear tissues [27]. The degree of tinnitus-related distress was reported to be positively correlated with cortisol levels [28]. The mechanical system of the significant decrease of C/D ratio in the honeybee group with the improvement of speech discrimination in noise remains unclear. However, the immunomodulatory power of the honeybee (Apis melifera) larvae activates the nonprimary auditory pathways, resulting in improving the speech perception in noise.
The DHEAS may potentially reduce the chronic inflammation,which has been thought to deteriorate the cochlear function [8]. In this study, the honeybee (Apis melifera) larvae significantly improved the speech discrimination in noise with reduction of the C/D ratio, however, no change in the inflammatory cytokine such as IL-6 and TNFα were found. The chronic inflammation may begin slowly, compatible with the temporal progression of presbycusis and chronic tinnitus [29]. Based on previous reports associated with age-related diseases, inflammaging may be an essential component in the pathophysiology of presbycusis, and the low-grade inflammation processes is strongly concerned with presbycusis [7, 9]. Also, the neuroinflammation-induced tinnitus has been associated with TNF-α production in the auditory cortex [11].
Oxidative stress accumulates damage induced by reactive oxygen species (ROS) and free radicals. Also, oxidative stress could be involved in presbycusis as well as other diseases associated with aging [30-33]. Although oxidized low-density lipoprotein is the most notable factor in atherosclerosis development, no effect of honeybee larvae on the MDA-LDL was found in this study. However, the serum level of the MDA-LDL in these participants was higher than the normal range in the population aged more than 55 years old (61-105 U/L). A recent animal study reported that the honeybee (Apis melifera) larvae increased nicotinamide in the NAD (nicotinamide adenine dinucleotide) pathway, and that the nicotinamide treatment reduced LPS-stimulated proinflammatory cytokines and attenuated the LPS-induced oxidative stress [34]. The honeybee (Apis melifera) larvae in our study may be contributed to improve speech discrimination in older adults suffering from hearing impairment through the NAD-related signaling mechanism.
Many studies have reported an association of hearing loss with cognitive impairment [35, 36]. Our results showed that the older adults with mild hearing loss were more responsive to the honeybee (Apis melifera) larvae intervention than older adults with moderate hearing loss. Early interventions of only lyophilized powder of enzymolized honeybee (Apis melifera) larvae and cognitive hearing training, listening to music, and hearing aids may improve speech discrimination in noise.
The intervention potentially improves the time processing ability of hearing, auditory memory, and auditory hearing in noise for older adults. Just as short-term auditory cognitive training was practical for hearing loss in older adults, the 12-week intervention in this study had no significant effect on pure-sound hearing levels but improved speech discrimination in noisy conditions. The subcortical neurotransmission of the auditory system by activating the prefrontal area may be involved in attention selection and the auditory stream separation decline with aging. In addition, the central auditory pathways are modulated by nonprimary auditory pathways such as the hypothalamus, reticular formation and limbic system [15, 36].
The 12-week intervention of honeybee (Apis melifera) larvae significantly improved speech discrimination in noise and reduced the visual analog scale for tinnitus loudness. The intervention of the honeybee (Apis melifera) larvae was related to reducing the C/D ratio. Recent studies demonstrated that the C/D ratio reflects critical effects on immune function, glucose metabolism, and memory [6, 37-40]. Negative feedback by the elevated C/D ratios in the HPA axis pathway promotes structural changes in the limbic system, especially hippocampal atrophy [41]. Changes in the limbic system of the hearing-impaired elderly may cause emotional instability and subjectively perceived tinnitus [42, 43]. We believe that the honeybee (Apis melifera) larvae have the potential to improve impaired speech discrimination in noise and self-perceived tinnitus for older adults with age-related hearing loss by modulating the endocrine pathways of the HPA system associated with auditory cognitive function-related centers.
The intervention of the honeybee (Apis melifera) larvae in our previous study decreased the threshold of the high-frequency hearing level [4, 44]. However, the improvement of the pure tone hearing levels in participants of the present study could not be found. The target population of the present study was older adults with age-related hearing loss, while the previous study included younger subjects with Meniere's disease and sudden deafness. No effect of the honeybee (Apis melifera) larvae on the pure tone hearing levels in the present study may be thus due to the pathological or age differences between both studies. This study has some limitations because it was composed of a limited number of subjects. Further studies on the lyophilized powder of enzymolized honeybee (Apis melifera) larvae are required to clarify the precise mechanism of therapeutic action in older adults suffering from hearing impairment and tinnitus.
REFERENCES
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, et al. (2003). Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol. 42:289-294.
- Torrente MC, Vergara R, Moreno-Gomez FN, Leiva A, San Martin S, et al. (2022). Speech Perception and Dichotic Listening Are Associated With Hearing Thresholds and Cognition, Respectively, in Unaided Presbycusis. Front Aging Neurosci. 14:786330.
- Humes LE. (1996). Speech understanding in the elderly. J Am Acad Audiol. 7:161-167.
- Aoki M, Wakaoka Y, Hayashi H, Kuze B, Mizuta K, et al. (2012). Effect of Lyophilized Powder Made From Enzymolyzed Honeybee Larvae on Tinnitus-Related Symptoms, Hearing Levels, and Hypothalamus-Pituitary-Adrenal Axis-Related Hormones. Ear and Hearing. 33:430-436.
- Vardanis A. (1967). Glycogen synthetase of bee larvae. J Biol Chem. 242:2306-2311.
- Mocking RJ, Pellikaan CM, Lok A, Assies J, Ruhe HG, et al. (2015). DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology. 59:91-101.
- Hunt KJ, Walsh BM, Voegeli D, Roberts HC. (2010). Inflammation in aging part 1: physiology and immunological mechanisms. Biol Res Nurs. 11:245-252.
- Verschuur C, Agyemang-Prempeh A, Newman TA. (2014). Inflammation is associated with a worsening of presbycusis: evidence from the MRC national study of hearing. Int J Audiol. 53:469-475.
- Verschuur CA, Dowell A, Syddall HE, Ntani G, Simmonds SJ, et al. (2012). Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire Ageing Study. Age Ageing. 41:92-97.
- Song C, Dinan T, Leonard BE. (1994). Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Disord. 30:283-288.
- Wang W, Zhang LS, Zinsmaier AK, Patterson G, Leptich EJ, et al. (2019). Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol. 17:e3000307.
- Tan K, Hu ZW, Chen WW, Wang ZW, Wang YC, et al. (2013). Fearful Foragers: Honey Bees Tune Colony and Individual Foraging to Multi-Predator Presence and Food Quality. Plos One. 8.
- Ventry IM, Weinstein BE. (1982). The hearing handicap inventory for the elderly: a new tool. Ear Hear. 3:128-134.
- Chermak GD, Musiek FE. (1997). Neurobiology of the central auditory nervous system relevant to central auditory processing. Chermak GD, Musiek FE, editors. San Diego: Singular Publishing Group.
- Humes LE, Watson BU, Christensen LA, Cokely CG, Halling DC, et al. (1994). Factors associated with individual differences in clinical measures of speech recognition among the elderly. J Speech Hear Res. 37:465-474.
- Kell AJE, McDermott JH. (2019). Invariance to background noise as a signature of non-primary auditory cortex. Nat Commun. 10:3958.
- Khalighinejad B, Herrero JL, Mehta AD, Mesgarani N. (2019). Adaptation of the human auditory cortex to changing background noise. Nat Commun. 10:2509.
- Patel P, Khalijhinejad B, Herrero JL, Bickel S, Mehta AD, et al. (2022). Improved Speech Hearing in Noise with Invasive Electrical Brain Stimulation. J Neurosci.
- Gordon-Salant S, Fitzgibbons PJ. (1997). Selected cognitive factors and speech recognition performance among young and elderly listeners. J Speech Lang Hear Res. 40:423-431.
- Souza PE, Boike KT, Witherell K, Tremblay K. (2007). Prediction of speech recognition from audibility in older listeners with hearing loss: effects of age, amplification, and background noise. J Am Acad Audiol. 18:54-65.
- Baulieu EE, Robel P. (1998). Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 95:4089-4091.
- Armanini D, Vecchio F, Basso A, Milone FF, Simoncini M, et al. (2003). Alzheimer's disease: pathophysiological implications of measurement of plasma cortisol, plasma dehydroepiandrosterone sulfate, and lymphocytic corticosteroid receptors. Endocrine. 22:113-118.
- de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, et al. (2016). Dehydroepiandrosterone, Its Sulfate and Cognitive Functions. Clin Pract Epidemiol Ment Health. 12:24-37.
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 30:65-91.
- Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, et al. (2016). Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrinology. 72:139-146.
- Aoki M, Okuda H, Ishihara H, Hayashi H, Ohashi T, et al. (2021). Hearing loss is associated with hippocampal atrophy and high cortisol/dehydroepiandrosterone sulphate ratio in older adults. Int J Audiol. 60:293-299.
- Rarey KE, Gerhardt KJ, Curtis LM, ten Cate WJ. (1995). Effect of stress on cochlear glucocorticoid protein: acoustic stress. Hear Res. 82:135-138.
- Hebert S, Lupien SJ. (2007). The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci Lett. 411:138-142.
- Weber C, Arck P, Mazurek B, Klapp BF. (2002). Impact of a relaxation training on psychometric and immunologic parameters in tinnitus sufferers. J Psychosom Res. 52:29-33.
- Roth TN. (2015). Aging of the auditory system. Handb Clin Neurol. 129:357-373.
- Sevindik M, Akgul H, Pehlivan M, Selamoglu Z. (2017). Determination of therapeutic potential of Mentha longifolia ssp. longifolia. Fresen Environ Bull. 26:4757-4763.
- Mohammed FS, Kına E, Sevindik M, Doğan M, Pehlivan M. (2021). Antioxidant and antimicrobial activities of ethanol extract of Helianthemum salicifolium (Cistaceae). Indian Journal of Natural Products and Resources. 12:459-462.
- Uysal İ, Mohammed FS, Şabik AE, Kına E, Sevindik M. (2021). Antioxidant and Oxidant status of medicinal plant Echium italicum collected from different regions. Turkish Journal of Agriculture-Food Science and Technology. 9:1902-1904.
- Yokoyama Y, Shinohara K, Kitamura N, Nakamura A, Onoue A, et al. (2021). Metabolic Effects of Bee Larva-Derived Protein in Mice: Assessment of an Alternative Protein Source. Foods. 10(11): 2642.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, et al. (2017). Dementia prevention, intervention, and care. Lancet. 390:2673-2734.
- Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, et al. (2009). Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 47:693-703.
- Wisniewski TL, Hilton CW, Morse EV, Svec F. (1993). The relationship of serum DHEA-S and cortisol levels to measures of immune function in human immunodeficiency virus-related illness. Am J Med Sci. 305:79-83.
- Herman WA, Senko A, Korczowska I, Lacka K. (2009). [Could serum DHEA and DHEAS levels be good risk predictors of metabolic syndrome and osteoporosis in the population of ageing men?]. Pol Merkur Lekarski. 27:197-201.
- Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, et al. (2010). Cortisol, DHEAS, their ratio and the metabolic syndrome: evidence from the Vietnam Experience Study. Eur J Endocrinol. 162:919-923.
- Vuksan-Cusa B, Sagud M, Mihaljevic-Peles A, Jaksic N, Jakovljevic M. (2014). Metabolic syndrome and cortisol/DHEAS ratio in patients with bipolar disorder and schizophrenia. Psychiatr Danub. 26:187-189.
- Farooqi NAI, Scotti M, Yu A, Lew J, Monnier P, et al. (2019). Sex-specific contribution of DHEA-cortisol ratio to prefrontal-hippocampal structural development, cognitive abilities and personality traits. J Neuroendocrinol. 31:e12682.
- Leaver AM, Seydell-Greenwald A, Rauschecker JP. (2016). Auditory-limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear Res. 334:49-57.
- Gunbey HP, Gunbey E, Aslan K, Bulut T, Unal A, et al. (2017). Limbic-Auditory Interactions of Tinnitus: An Evaluation Using Diffusion Tensor Imaging. Clin Neuroradiol. 27:221-230.
- Aoki M, Fukushima S, Ohkuma A, Tatefuji T. (2015). Significant symptomatic benefit of the enzymolyzed honeybee larvae for patients with mild self-perceived tinnitus handicap: A double-blind placebo-controlled trial. Jpn Pharmacol Ther. 43:507-514.
 Abstract
Abstract  PDF
PDF