Past Issues
Review on Phytochemistry, Medicinal Properties, and Toxicities of the Genus Musa
Tesfa Begashaw1, Abebe Dagne2, Desalegn Yibeltal2,*
1Debre Birhan University, College of Medicine and Health Sciences, Ethiopia
2Debre Markos University, College of Medicine and Health Sciences, Ethiopia
*Corresponding Author: Desalegn Yibeltal, Debre Markos University, College of Medicine and Health Sciences, Ethiopia; Email: [email protected]
Received Date: June 18, 2023
Publication Date: July 22, 2023
Citation: Begashaw T, et al. (2023). Review on Phytochemistry, Medicinal Properties, and Toxicities of the Genus Musa. Traditional Medicine. 4(2):17.
Copyright Begashaw T, et al. © (2023).
ABSTRACT
Herbal medicines are extensively used in developed and developing countries for primary healthcare systems because of their wide biological and medicinal activities, higher safety margins, and lesser costs.
The purpose of this review is to organize information about traditional medicine, morphology, phytochemicals, toxicological, and pharmacological reports of the prominent species of Musa (Musaceae) from different studies. This review was done by organizing different information from different articles in the form of PDF that were obtained via PubMed, Sci-Finder, Science Direct, and Google Scholar.
The major finding in this review is that the genus Musa is a veritable source for drug bioprospecting that will be of benefit to scientific research and pharmaceutical industries.
The genus comprises seventy-one species in which flavonoids, saponins, cardiac glycoside, phenolic acids, and alkaloids are the major classes of phytoconstituents. Species of this genus have traditional medicinal value; among these M. sapientum, M. paradisiaca, M. balbisiana, M. troglodytarum, M. acuminata, and others are extensively used as traditional medicine in tropical and subtropical countries.
Keywords: Musa, Ethnopharmacology, Phytochemicals, Pharmacology, Toxicology
INTRODUCTION
Treatment of disease using medicinal plants is as old as mankind itself. In ancient times, humans used medicinal plants simply via decoction, maceration, and infusion. Such a tradition of practice is continued from generation to generation mainly through word of mouth (Petrovska, 2012) [1].
Recently, WHO proposed that 80% of the population around the world depends on traditional medicine systems either partially or totally for their primary healthcare needs. This form of treatment is called ethnomedicine (M. Fawzi Mahomoodally, 2013) [2].
Currently, there is a consensus about the use of medicinal plants and traditional health care systems in solving the health care problems, efficacy and safety of medicinal plants in curing various diseases. This is Because of growing use of traditional medicine (Mohammed Kemal Hossain, 2011) [3].
In the future medicinal plants are a hope for human beings in the drug development process, health promotion, and treatment of diseases, because there are a lot of plants around the world that are not well investigated. Use of traditional remedies increases when conventional medicine is ineffective in treatment of disease such as advanced cancer and infectious disease (Mani, 2017) [4].
Based on the International Union for Conservation of Nature, there are 50,000-80,000 plant species used for medicinal purposes around the world. Medicinal plants are used in herbal remedies, but they rapidly disappear at high speed (Chen et al., 2016) [5].
Among those species, 71 belong to the genus Musa, which has various health benefits (Hastuti et al., 2019) [6]. Among the species of Musa, M. sapientum (Muz in Amharic) is found in Ethiopia which is used as abortion medicine. M. paradisiaca (Musi in Oromiffa) is also another species found in Ethiopia, which is highly used as traditional medicine (Admasu Moges & Yohannes Moges, 2020) [7].
A brief review of 80 articles that were written in English through electronic databases like PubMed, Sci-Finder, Science Direct, and Google Scholar was conducted. We use articles for this review that were PDF and with authors, unless and otherwise we didn’t not include. Chemdraw software was used to draw the structure of compounds. This review includes the common species' ethnopharmacological, phytochemistry, medicinal properties, and toxicities of species from the genus Musa.
THE GENUS MUSA
It is the genus of flowering plants grouped under the family Musaceae, which is an angiosperm. It is also one of the most important crops in several countries as a source of enriched food, being responsible for up to 15% of total fresh fruit production and the fourth most relevant crop worldwide after rice, wheat, and maize (Sharrock, 1998) [8].
It has four major groups, which include both seeded and non-seeded types. Two of groups (Callimusa and Australimusa) contain species with a chromosome number of 2n=20 and they are nonseeded, but the remaining two groups (Eumusa and Rhodochlamys) contain species with a basic chromosome number of 2n=22 and they are seeded (Daniells etal., 2001) [9].
GEOGRAPHICAL DISTRIBUTION OF THE GENUS MUSA
The genus Musa is distributed in the humid tropics and subtropics countries. Of these America, Africa, Asia, Europe, and Australia (Vilhena et al., 2019) [10].
In the tropics, it is distributed from 175° East to 1500 West longitude. Also from 300 North to 230 South latitude (Nayar, 2010) [11].
It is also distributed in some tropical rainforests, wet evergreen forests. Also in the deciduous forests of low rainfall in India (Subbaraya, 2006) [12].
Musa is also distributed in Uganda, Rwanda, Burundi, and Tanzania. In these countries it is distributed at altitudes of 1066-1111m (M.Kmira et al., 2016) [13] (Table 1 Figure 1).
Table 1: Geographical distribution of the common Musa species.
|
Species name |
Geographical distribution |
Reference |
|
M. sapientum |
North America, Southeast Asia, India, Mediterranean, Tropical Africa, Subtropical Africa |
(Dixit et al., 2014) [14] |
|
M. paradisiaca |
India, Burma, Tropical Africa, Egypt, Southern Japan, South Brazil |
(Galani, 2019) [15] |
|
M. borneensis |
Indonesia |
(Sunandar & Kurniawan, 2020) [16] |
|
M. balbisiana |
India, Tropical Africa, Southeast Asia, Australia |
(Pollefeys et al., 2004) [17] |
|
M. acuminata |
Indonesia, Sulawesi |
(Hastuti et al., 2019) [6] |
|
M. ornata |
Southeast Asia, Bangladesh, Burma, India |
(Häkkinen & Sharrock, 2002) [18] |
|
M. schizocarpa |
Indonesia, Papua New Guinea |
(Lentfer, 2009) [19] |
|
M. textilis |
Southeast Asia, Pacific Islands |
(Hapsari, 2014) [20] |
|
M. dasycarpa |
India, Myanmar |
( Häkkinen & Väre, 2008) [21] |
Figure 1: Geographical distribution of the genus Musa (Kennedy, 2009) [22].
 BOTANICAL DESCRIPTION OF THE GENUS MUSA
BOTANICAL DESCRIPTION OF THE GENUS MUSA
In the natural habitat, the genus Musa has 25-30 suckers in vertical position.
A mature pseudostem of genus Musa has a height of 1.1 m with a diameter of 5 cm at the base which makes it swollen. Also, it has a petiole of 25 cm long Häkkinen & V̈are, 2008) [23].
The bract and flowers of Musa are inserted independently on the peduncle. Also, it has flowers attached to the axial of the bracts, and the basal flowers are either female or male (found at distal hands) (Sulistyaningsih, 2016) [24] (Figure 2).
A B C
Figure 2: Picture showing M. sapientum at fruit stage(A),picture showing M. paradisiaca at fruit and flowering stage (B), and picture showing M. troglodytarum at flowering stage (C) (Nelson et al., 2006) [25].
ETHNO-PHARMACOLOGICAL USE OF THE GENUS MUSA
In both tropical and subtropical countries, the flower of genus Musa is traditionally used to treat ulcers, dysentery, and bronchitis.
Also cooked flowers are good food for diabetic patients (Ajijolakewu et al., 2021) [26].
In subtropical countries the methanolic, and acetone extractions of the fruits of the genus Musa is traditionally used for diarrhea, dysentery, intestinal lesions in ulcerative colitis, diabetes, uremia, nephritis, gout, hypertension, cardiac disease, and excess menstruation disorder. Also the leaf extracts used in eczema as a cool dressing for blisters and burns (Imam & Akter, 2011) [27].
In Ethiopia, the oozing fluid from the stem of M. paradisiaca is important for wound healing. It is also important for coagulation and secondary infection (Tuasha et al., 2018) [28].
In Ethiopia, M. paradisiaca is important for the management of diarrhea as traditional medicine. It is Also used for ulcers and bronchitis (Kidane et al., 2014) [29].
In Ethiopia, Musa sapientum (Muz in Amharic) is traditionally used for abortion. It is also used for blisters and ulcers (Admasu & Yohannes, 2020) [7].
In Asia, the root extract of the genus Musa is used as traditional medicine for the management of inflammatory. Also the leaf extracts of the genus Musa is used for helminthic complications (Oguntibeju, 2019) [30].
The root extracts of the genus Musa, specifically M. balbisiana traditionally used as antilipidemic agents. The root, and shoot extract of M. balbisiana is also traditionally used for diabetic (Kalita et al., 2016) [31].
The fruit extracts of the genus Musa are traditionally used for laxative purposes, constipation in children, and the extracts of the core of the stem are also important for dissolving the stones in the kidney as well as in the urinary bladder. In addition to this, the mixture of the fruit extracts of Musa with coconut oil is used for flushing urinary blocks (Bandaranayake, 1998) [32].
In Korea, the air-dried powdered aqueous extracts of the leaves and rhizomes of the genus Musa are traditionally used as anti-TB. It is also used against MDR-TB (Molina-Salinas et al., 2019) [33].
In the Philippines, the extracts of leaves of Musa basjoo which have natural fibers are traditionally used to decrease their weight. It also has mild laxative properties (Lacuna-Richman, 2002) [34].
The aqueous extract of the fruit of M. paradisiaca is used for hair growth-promoting activity. It is also used for epilepsy, leprosy, hemorrhages, renal calculi, hysteria, and analgesic (Mahadeva Rao et al., 2014) (Table 2) [35].
Table 2: Phytochemicals of the genus Musa with their ethnopharmacological use.
|
Phytochemicals |
Ethno pharmacological use |
|
Tannic acid |
Used for the treatment of burns |
|
Catechin |
Enables LDL to oxidation |
|
Gallic acid |
Antioxidant and hepatoprotective |
|
Cinnamic acid |
As sweetener |
|
coumaric acid |
Antioxidant and reduce the risk of stomach ache |
|
Quercetin |
Promote cardiovascular health by encouraging blood flow |
|
Ferulic acid |
Antioxidant, antimicrobial, anti-inflammatory, antiallergic, and anticancer |
|
Carotene |
Reduce the risk of CVD and cancer |
|
Violaxanthin |
Used as a food colorant |
|
Cryptoxanthin |
Reduce the risk of lung cancer |
|
Serotonin |
Increase wellbeing and happiness |
|
Catecholamines |
Used to increase blood pressure, glucose level, heartbeat rate |
|
Sitosterol |
Used in the management of benign prostate hyperplasia |
|
Campesterol and stigmasterol |
Used for the reduction of cholesterol absorption |
PHYTOCHEMICAL COMPOSITION OF THE GENUS MUSA
The most common phytochemically investigated Musa species are M. sapientum, M. paradisiaca, M .assamica, M. aurantiaca, M. acuminate, M. balbisiana, M.basjoo, M. textilis, M. troglodytarum, M. brachycarba, M. cavendishii, M. cavandanaish and M. saba. Major Phytochemicals are alkaloids, saponins, cardiacglycosides, flavonoid, and quercetin 3-O rhamnosyl galactosides (20) are majorly investigated (Obiageli et al., 2016) [36].
Other common phytochemically investigated chemicals from the genus Musa is anthocyanins, lutein, α-, β- carotenes, neoxanthin, cryptoxanthin, coumarin, β-sitosterol. Also terpenes are other common phytochemicals that are investigated from the genus Musa (Oyeyinka & Afolayan, 2020) [37].
In addition to the above major phytochemicals, M. sapientum contains carbohydrates, norepinephrine, serotonin, and dopamine, tryptophan, indole compound, and pectin. M. paradisiaca contains serotonin, norepinephrine, tryptophan, indole compounds, starch, iron, vitamin C, vitamin B, albuminoids, fats, and mineral salts (Imam & Akter, 2011) [27].
The percentage content for tannin or flavan-3-ol (9.13%), alkaloid (8.16%), flavonoids other than tannin (4.02%), saponin (3.5%), cardiac glycoside (1.6%), oxalate (0.162%). Also hemagglutinin (1.8814 mg/kg), phytate (1.2967 mg/kg), phenols (5.5743 mg/kg) (Onyema et al., 2016) [38].
FLAVONOIDS
Flavonoids are active secondary metabolites that enable the genus Musa to have antioxidative, antidiabetic, and Anti-Alzheimer. Also it enables the genus Musa to have anti-inflammatory activities (Oresanya et al., 2020) [39].
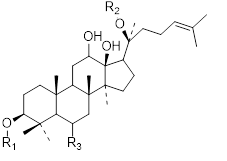
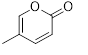
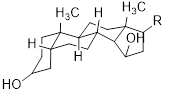
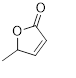
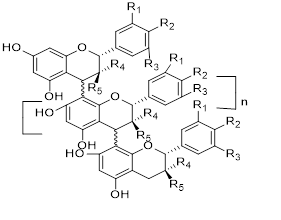
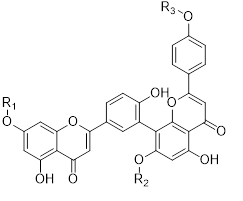
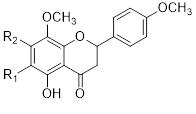
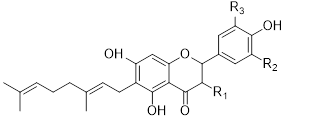
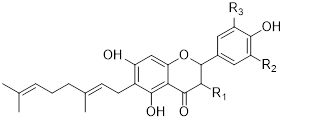
Flavonols (Quercetin 3-O rhamnosyl galactoside (20) ), anthocyanidins (cyanidin 3-O glucoside (21), delphinidin (14), peonidin (16), malvidin (17), pelargonidin (15)), flavones heveaflavone (5), Amentoflavone-7, 4-dimethyl Ether (6), Podocarpus flavone (7), flavanols ((+)-catechin (1), (-)-epicatechin (2), (+)-gallocatechin (3), (-)-epigallocatechin (4), isoflavones (daidzein (22), flavanones (naringenin (19)). Also chalcones (18) are the major flavonoids extracted from the aerial parts of the genus Musa (Dong et al., 2016) [40].
Flavonoids can be extracted from the aerial parts of the genus Musa. These can be performed via the decoction method using distilled water, then allow the decoction product to be filtered and stored at 4°c (Lino et al., 2011) (Table 3) [41].
Table 3: Favonoids isolated from the species of genus Musa (Lino et al., 2011) [41].
|
No. |
Name of the compound |
Species |
Plant parts |
||||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
Class of compound |
||||
|
1 |
Catechin |
OH |
OH |
H |
H |
OH |
Condensed tannin |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
2 |
Epicatechin |
OH |
OH |
H |
OH |
H |
Condensed tannin |
M.paradisiaca M.sapientum M.balbisiana M.acuminat M.troglodytarum |
Aerial parts |
|
3 |
Gallocatechin |
OH |
OH |
OH |
H |
OH |
Condensed tannin |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.droglodytarum |
Aerial parts |
|
4 |
Epigallocatechin |
OH |
OH |
OH |
OH |
H |
Condensed tannin |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
No. |
Name of the compound |
Species |
Plant parts |
||||
|
R1 |
R2 |
R3 |
Class of compound |
|
|||
|
5 |
Heveaflavone |
CH3 |
CH3 |
CH3 |
Flavone |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
6 |
Amentoflavone -7,4-dimethyl Ether |
H |
CH3 |
CH3 |
Flavone |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
7
|
Podocarpus flavone |
H
|
H H |
CH3
|
Flavone |
M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
No. |
Name of the compound |
Species |
Plant parts |
||||
|
R1 |
R2 |
R3 |
Class of compound |
||||
|
10 |
3-o methyl-5-hydroxy Diplacone |
H |
OMe |
OH |
Flavanone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
11 |
3-O-methyl-5-O-methyl diplacone |
H |
OMe |
OMe |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
12 |
Mimulone |
H |
H |
H |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
13 |
Diplacone |
H |
OH |
H |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
No. |
Name of the compound |
Species |
Plant parts |
||||
|
R1 |
R2 |
R3 |
Class of compound |
||||
|
10 |
3-o methyl-5-hydroxy Diplacone |
H |
OMe |
OH |
Flavanone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
11 |
3-O-methyl-5-O-methyl diplacone |
H |
OMe |
OMe |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
12 |
Mimulone |
H |
H |
H |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
|
13 |
Diplacone |
H |
OH |
H |
Flavone |
M.paradisiaca M.sapientum M.acuminata M.balbisiana M.troglodytarum |
Aerial parts |
Chalcones (18), naringenin (19), quercetin 3-O-rhamnosyl galactoside (20), cyanidin-3-O glucoside chloride (21), daidzein (22), and petunidin (23) (Figure 3).
Figure 3: Structural formula of flavonoids isolated from the species of genus Musa.
SAPONINS
Saponins are secondary metabolites that enable the genus Musa to have antiulcer activity, wound healing activity, antidiabetic activity, an antidote for Crotalidae venoms, antilithiatic activity. Also it enables the genus Musa to have skeletal muscle contraction activities (Jayakumari et al., 2018) [42].
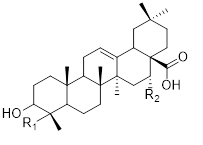
Saponins are extracted from the aerial parts of the genus Musa. These can be performed via maceration using 70% ethanol solvent Steroid and triterpenoid saponins are the major classes of saponins found in the genus Musa (Nofianti et al., 2021) [43] (Table 4).
Table 4: Saponins isolated from the species of genus Musa (Nofianti et al., 2021) [43].
|
No. |
Name of the compound |
Species |
Plant parts |
||||
|
R1 |
R2 |
R3 |
Class of compound |
||||
|
24 |
Protopanaxadiol |
Sugar |
Sugar |
H |
Steroidal saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
|
25 |
Protopanaxatriol |
H |
Sugar |
O-sugar |
Steroidal saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
|
No. |
Name of the compound |
Species |
Plant parts |
|||
|
R1 |
R2 |
Class of compound |
||||
|
26 |
Gypsogenin |
CHO |
H |
Triterpenoid saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
|
27 |
Gypsogenic acid |
COOH |
H |
Triterpenoid saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
|
28 |
Quillaic acid |
CHO |
OH |
Triterpenoid saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
|
29 |
Hederagenin |
CH2OH |
H |
Triterpenoid saponin |
M.acuminata M.paradisiaca M.balbisiana M.saba M.sapientum |
Aerial parts |
CARDIAC GLYCOSIDES
Cardiac glycosides are secondary metabolites that enable the genus Musa majorly to treat congestive heart failure. It is also enables the genus Musa to treat cardiac arrhythmias. (Nascimento, 2019) [44].
Cardiac glycosides are isolated by soaking the air-dried powdered material of the genus Musa. These can be performed by using 70% alcohol for 2 hours and then filtered. Then lead acetate is added to the filtrate to precipitate out resin, tannin, and pigments, and then add distilled water and then filter. And many other further purification processes take place.
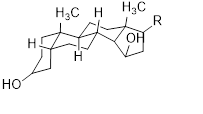
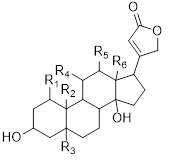
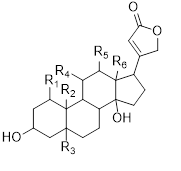
Major cardiac glycosides found in the genus Musa are cardenolide (30), bufadienolide (31), isocardenolide (32), digitoxigenin (33), digoxigenin (34), gitoxigenin (35), strophanthidin (36). Also ouabagenin (37) (Sahaa et al., 2013) (Table 5) [45].
Table 5: Cardiac glycosides isolated from the species of genus Musa (Sahaa et al., 2013) [45].
|
No. |
Name of the compound |
Species |
Plant parts |
||
|
R |
class of compound |
||||
|
30 |
Cardenolide |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
|
31 |
bufadienolide |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
|
No. |
Name of the compound |
species |
Plant parts |
||||||||
|
R |
Class of compound |
||||||||||
|
32 |
Isocardenolide |
|
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
||||||
|
|
|
|
|
||||||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Class of compound |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|||
|
33 |
Digitoxigenin |
H |
CH3 |
H |
H |
H |
CH3 |
Cardiac glycoside |
|||
|
34 |
Digoxigenin |
H |
CH3 |
H |
H |
OH |
CH3 |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
|
No. |
Name of the compound |
Species |
Plant parts |
|||||||
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
Class of compound |
|
|
||
|
35 |
Gitoxigenin |
H |
CH3 |
H |
H |
H |
CH3 |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
36 |
Strophanthidin |
H |
CHO |
OH |
H |
H |
CH3 |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
|
37 |
Ouabagenin |
OH |
CH2OH |
OH |
OH |
H |
CH3 |
Cardiac glycoside |
M.cavendishii M.paradisiaca M.sapientum M.balbisiana M.acuminata M.troglodytarum |
Aerial parts |
ALKALOIDS
Alkaloids are active secondary metabolites that enable the genus, Musa, to have antibacterial, antispasmodic, stimulant, anesthetic, anticancer, and analgesic effects. Many alkaloids in the genus Musa are extracted from leaves and constituents for most drugs (Dele et al., 2019) [46].
Alkaloids can be extracted by soaking the dried powdered leaves or flowers of the genus Musa. These can be performed by using ethanol or methanol for 48 hours with continuous shaking and can be purified with acid-base extraction methods (Asuquo & Udobi, 2016) [47].
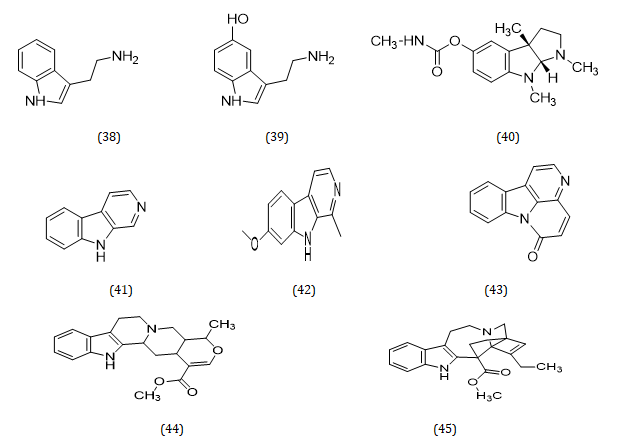
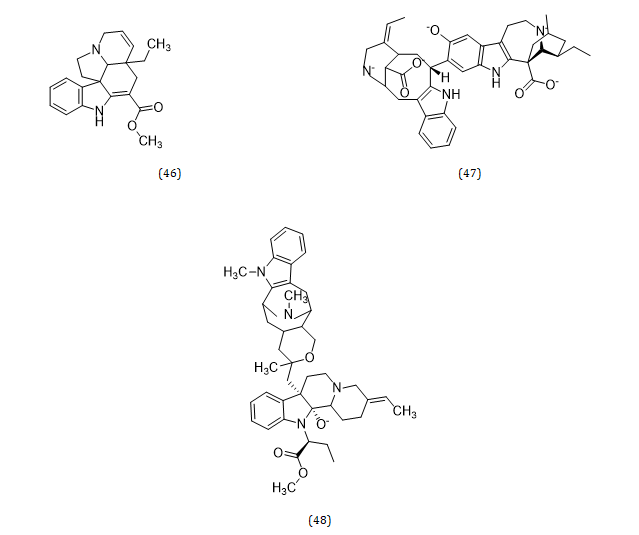
Indole alkaloids are the major alkaloids with therapeutic importance in the genus Musa. These include Tryptamine (38), Serotonin (39), Physostigmine (40), β-carboline (41), Harmine (42), Canthinone (43), Ajmalicine (44), Catarantine (45), Tabersonine (46), Voacamine (47), and Villalstonine (48) (Hamid et al., 2017) (Figures 4, 5) [48].
Figure 4: Structural formula of alkaloids isolated from the species of genus Musa.
Figure 5: Structural formula of alkaloids isolated from the species of genus Musa.
PHENOLIC ACIDS
Phenolics are active secondary metabolites that enable the genus Musa is majorly to have antifungal, antioxidant. Also enables the genus Musa to have antibacterial activities (Peel et al., 2019) [49].
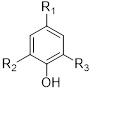
Hydroxybenzoic acid derivatives (gallic acid (49), vanillic acid (52), syringic acid (53), hydroxycinnamic acid derivatives (p-coumaricacid (51), caffeic acid (50), are the major phenolic acids extracted from the genus Musa (Oyeyinka & Afolayan, 2020) [37].
Phenolics can be extracted by soaking fresh or dried material of the genus Musa. These can be performed by using the solvent hexane, chloroform, and 80% methanol (v/v) (K.Phillips et al., 2011) [50] (Table 6).
Table 6: phenolic acids isolated from the species of genus Musa (K.Phillips et al., 2011) [50].
|
No. |
Name of the compound |
Species |
Plant parts |
||||
|
R1 |
R2 |
R3 |
Class of compound |
||||
|
49 |
Gallic acid |
CO2H |
OH |
OH |
Phenolic acids |
M.sapientum M.paradisiaca M.acuminata M.troglodytarum M.balbisiana |
Aerial parts |
|
50 |
Caffeic acid |
CH=CH-CO2H |
OH |
H |
Phenolic acids |
M.sapientum M.paradisiaca M.acuminata M.troglodytarum M.balbisiana |
Aerial parts |
|
51 |
P-coumaric acid |
CH=CH-CO2H |
H |
H |
Phenolic acids |
M.sapientum M.paradisiaca M.acuminata M.troglodytarum M.balbisiana |
Aerial parts |
|
52 |
Vanillic acid |
CO2H |
H |
OCH3 |
Phenolic acid |
M.sapientum M.paradisiaca M.acuminata M.troglodytarum M.balbisiana |
Aerial parts |
|
53 |
Syringic acid |
CO2H |
OCH3 |
OCH3 |
Phenolic acid |
M.sapientum M.paradisiaca M.acuminata M.troglodytarum M.balbisiana |
Aerial part |
MISCELLANEOUS COMPOUNDS ISOLATED FROM THE GENUS MUSA
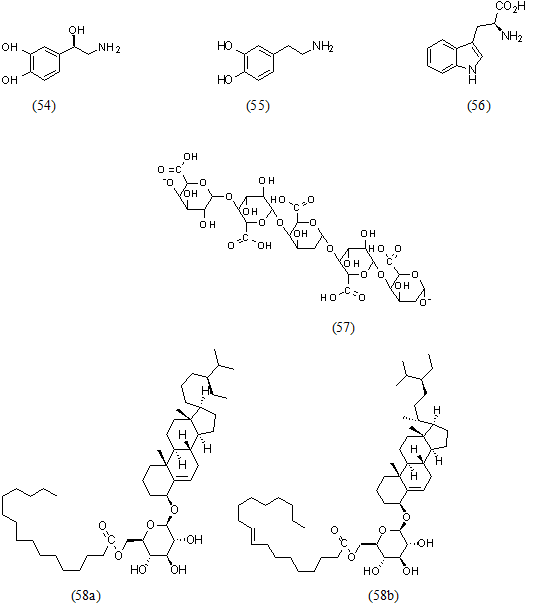
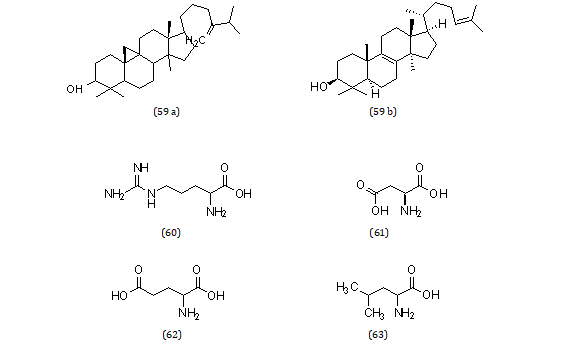
Norepinephrine (54), dopamine (55), L-tryptophan (56), pectin (57), acyl steryl glycosides (58), triterpenes (59), arginine (60), aspartic acid (61). And also glutamic acid (62), leucine (63) (Md.Saidul Islam, 2013) [51] (Figures 6,7).
Figure 6: Structural formula of miscellaneous compounds isolated from the species of genus Musa.
Figure 7: Structural formula of miscellaneous compounds isolated from the species of genus Musa.
PHARMACOLOGICAL REPORTS FROM THE GENUS MUSA SPECIES
Pharmacological studies have been reported from different Musa species which are important as medicinal plants. According to my literature search from 71 Musa species, pharmacological reports are mostly available for M. sapientum, M. paradisiaca, M. acuminata, M. troglodytarum, M. cavendishii, M. saba and M. balbisiana, M. basjoo, M. ornata, M. schizocarpa, M. textilis, and M. dasycarpa. Pharmacological activities such as anticancer, antihypertensive, antimicrobial, antidiabetic, antiulcer, diuretic, and other below-mentioned activities are reported from this genus.
ANTICANCER ACTIVITY
Based on in vitro studies of the aqueous methanol extracts, ethanol extracts, and hexane extracts of M. sapientum, M. paradisiaca, M. cavendishii, M. acuminata, and M. balbisiana peel, fruit, leaf, and stem show significant anti-tumor activity. It is against MCF-7 breast cancer, cervical cancer, colon cancer, liver cancer, oral cancer, prostate cancer, and skin cancer via concentration-dependent apoptosis (Mondal et al., 2021) [52].
In vivo anticancer activity of methanolic and ethanolic extracts of the flower from M. acuminata was investigated in male rats. It is performed with minimum and maximum inhibitory concentrations of 200 mg/kg and 500 mg/kg respectively (Liu et al., 2018) [53].
ANTIHYPERTENSIVE ACTIVITY
A study was done on 20 respondents with stage 1 and stage 2 hypertension who are characterized by gender, age, and working area in Karanganyar, after an intervention with potassium-rich Musa species (M. paradisiaca. M. acuminata and M. cavendishii) results in inhibition of the renin-angiotensin system also decreases aldosterone secretion, then reabsorption of sodium and water in the kidney tubules are decreased. After that diuresis is increased and blood pressure is decreased. According to this study recommended potassium intake is 351 mg/day which can be fulfilled by the above species of genus Musa (435 mg/day) (Susanti et al., 2019) [54].
ANTIMICROBIAL ACTIVITY
An acetone extracts of the leaves of M. cavendishii (IC50=53 microgram/ML), M. acuminata (IC50=61 microgram/ML) and M. saba (IC50=99 microgram/ML) show strong inhibitory activity against gram-positive bacteria (Bacillus cereus, Micrococcus luteus, Staphylococcus aureus, Streptococcus faecalis). Also against gram-negative bacteria (Aeromonashydrophila, Escherichia coli, Salmonella enterica, Shigellasonnei). Also, two ethanol extracts of leaves of (M. cavendishii and M. acuminata) show strong inhibitory activity against B. cereus (Jouneghani et al., 2020) [55].
ANTIDIABETIC ACTIVITY
Ethanolic extracts of M. paradisiaca, M. sapientum, M. acuminata, and M. balbisiana leaves, and peel show lowering of blood glucose level at 500 mg/kg body weight in diabetic-induced rats. This study also shows an increase in protein and albumin with decreased serum urea and creatinine levels which plays a role in the management of renal dysfunction in diabetic causes (Edition SC, 2019) [56].
ANTIULCER ACTIVITY
In vivo antiulcer activity of aqueous and ethanolic extracts of peels of M. paradisiaca was studied in Wistar albino rats using ethanol, aspirin, indomethacin, and pyloric ligation-induced ulcer. The fresh peels of M. paradisiaca were extracted using hot water and then lyophilized. Standard drugs, distilled water, and extracts were injected intraperitoneally before inducing ulcers. Lethality tests and quantitative phytochemical analysis were carried out using standard methods. Results showed that the extract at 50, 100, and 200 mg/kg has 73.87, 80.18, and 81.98% protection respectively against ethanol-induced ulcers, but cimetidine (50 mg/kg) has 72.07% ulcer protection benefits. Also, the extract inhibits aspirin-induced ulcers. Similar to cimetidine, the extract does not inhibit indomethacin-induced ulceration. But the extracts at 50, 100, and 200 mg/kg and cimetidine at 50 mg/kg inhibit pyloric ligation-induced ulcers by 100 and 75% respectively. This study suggests the antiulcerogenic ability of the extract supports ethnomedicinal use as an antiulcer. Up to 5000 mg/kg of the extract does not cause mortality of animals; this indicates the safety of the extract. The extract was rich in flavonoids (1.40 ± 0.02 mg/100 g) (Chinwe N et al., 2014) [57].
DIURETIC ACTIVITY
In vivo diuretic activity of the aqueous extracts of the leaves from M. sapientum, M. paradisiaca and M. acuminate show an increase in urine volume and K+ as well as other electrolytes excretion than normal saline in a study in rats. Continuous ethanolic extracts of M. sapientum also give this diuretic effect. Phytoconstituents like saponin, flavonoids, and terpenoids are responsible for this effect (Imam & Akter, 2011) [27].
ANALGESIC ACTIVITY
In vivo analgesic activity of the aqueous and ethanolic leaf extracts from M. sapientum and M. paradisiaca (both extracts at 400 mg/kg i.p.) significantly increases reaction time in the hot plate method in comparison to the vehicle-treated group. The maximum analgesic effect of M. sapientum extracts was observed at 2 hours (Barua & Das, 2013) [58].
ANTI-ALLERGIC ACTIVITY
The pseudo-stem powder of M. paradisiaca was suspended with acacia 10 mg/ML for experiments. Acute oral toxicities were seen in both sex rats. Female mice were sensitized against ovalbumin. Single-dose powder (60 mg/kg b.w.) or ketotifen preparation (3 mg/kg b.w.) were administered 1 hour before the induction system anaphylaxis by I.V. injection of ovalbumin. Overall the test product, (0, 6; 2; 6 and 20 mg/kg b.w. x per day) was given to mice during the immunization period, then followed by the induction of systemic anaphylaxis or the measurement of passive cutaneous anaphylaxis titers of mice antisera in rats. There were neither deaths nor any sign of toxicity among the rats treated with powder of M. paradisiaca. Unlike ketotifen preparation, a single oral dose of this product was unable to inhibit systemic anaphylaxis in mice. However, a daily oral treatment produced a significant reduction of active and passive anaphylaxis. This study suggests that a pseudo-stem powder of M. paradisiaca has an antiallergic ability (Garcia Mesa et al., 2019) [59].
HYPOLIPIDEMIC ACTIVITY
In vivo hypolipidemic activity of aqueous extracts of leaf from M. sapientum and M. paradisiaca were studied on hyperlipidemic-induced Wistar rats in Nigeria. Results showed that M. sapientum extract (100 mg/kg b.w.) has a lipid-lowering effect and reduce other cardiovascular complication similar to the standard drug atorvastatin (70 mg/kg b.w.) (C. Edenta et al., 2014) [60].
ANTILITHIATIC ACTIVITY
In vivo antilithiatic activity of methanolic extracts of powder from M. sapientum, M. paradisiaca and M. acuminata was studied on renal calculi induced albino rats in Asia, results showed that there was an observation of a significant decrease in the size of kidney stones under in vitro conditions. This is due to the presence of organic constituents like β-sitosterol, quercetin, tannins, and saponins. This result suggests that the above Musa species powder extract was a good natural formulation for kidney stones (Prasobh & Revikumar, 2014) [61].
ANTIDIARRHEAL ACTIVITY
In vivo antidiarrheal activity of aqueous and methanolic extracts of leaves from M. sapientum, pseudo-stem powder of M. paradisiaca and M. acuminate were studied on castor oil and magnesium sulfate-induced diarrheal mice in Bangladesh, the result showed that the extract (at doses of 100 mg/kg and 200 mg/kg of b.w. respectively) decrease the frequency and severity of diarrhea. Not only this but also the extract has the potential to decrease the frequency and severity of bacterial strain-induced diarrhea (Sarowar Hossain et al., 2011) [62].
MUSCLE RELAXANT ACTIVITY
In vivo muscle relaxant activity of aqueous and ethanolic extracts of leaves from M. sapientum, pseudostem powder of M. paradisiaca, and M. acuminata was studied on directly and indirectly evoked contractions of isolated phrenic nerve hemidiaphragm preparation of mouse in India, Result showed that the extract first augments both the evoked contractions then block them. The two major constituents responsible for this activity are potassium nitrate and magnesium nitrate (Barua & Das, 2013) [58].
WOUND HEALING ACTIVITY
In vivo wound healing activity aqueous extracts of leaves from M. paradisiaca was studied on streptozotocin (STZ 60 mg/kg via I.P)-induced diabetic rats (fasting) in Chinese, The application of topical super green ointment (SG 1%) obtained from M. paradisiaca result in decreasing of wound area in diabetic rats. This is also effective for burning wounds (Cheng et al., 2020) [63].
ECOLOGICAL USES
The genus Musa is important to maintain soil fertility via dust and young leaves cut from Musa tree has domestic uses, like conservation of food and meat. Also, it important to prevent soil erosion (Mapongmetsem et al., 2012) [64].
NUTRITIONAL VALUE
M. sapientum and M. paradisiaca (banana) are important food crop in the humid forest and mid altitude agro-ecological zones of sub Saharan Africa and one of the major source of carbohydrate in Asia, Oceania, Africa and central Americas. The edible part of M. sapientum and M. paradisiaca is the fruit (finger) that is formed at the maturity of rhizomes (Wariboko C. and Alamene A., 2017) [65].
TOXICOLOGY
In vivo toxicological study of aqueous extracts of dried powder from M. sapientum, M. paradisiaca and M. acuminata was done on Wistar albino rats in Nigeria for kidney and liver toxicities, the orally administered extracts from 2000-5000 mg/kg results in changes of ALT, AST, total protein and albumin which documented as hepatotoxic and nephrotoxic. At a dose of above 5000 mg/kg the rats died (LD50 >5000 mg/kg) (Chidi Edenta et al., 2017) [65].
CONCLUSION
The genus Musa is a veritable source for drug bioprospecting that will be of benefit to scientific research and pharmaceutical industries.
There are approximately 71 recorded species of the genus Musa worldwide, but only 18% of them have been studied for their medicinal properties, phytoconstituents, and toxicities.
This review demonstrates that 63 secondary metabolites have been isolated and identified from the genus Musa. Flavonoids (23), saponins (6), cardiac glycosides (8), alkaloids (11), phenolic acids (5), and other compounds (10). Almost all secondary metabolites are isolated from the aerial parts of the genus Musa while few are from other plant parts.
Among the genus Musa, most ethnopharmacological reports are available for M. sapientum, M. paradisiaca, M. acuminata, M. cavendishii, M. ornate, M. troglodytarum, M. assamica, M. aurantiaca, M. balbisiana, and M. saba. The reports on pharmacologic activities of the species and isolated compounds include: anticancer (M. sapientum, M. paradisiaca, M. acuminata and M. balbisiana), antihypertensive (M.paradisiaca, M. acuminata and M. cavendishii), antimicrobial (M. acuminata and M. cavendishii), antidiabetic (M. paradisiaca, M. sapientum, M. acuminata and M. balbisiana), antiulcer (M. paradisiaca), diuretic (M. sapientum, M. paradisiaca and M. acuminata), analgesic (M. sapientum and M. paradisiaca), antiallergic (M. paradisiaca), wound healing activity (M. paradisiaca), hypolipidemic (M. sapientum and M. paradisiaca), antiurolithiatic (M. sapientum, M. paradisiaca and M. acuminta), antidiarrheal (M. sapientum, M. paradisiaca and M .acuminta) and muscle relaxant (M. sapientum, M. paradisiaca and M. acuminta). Toxicities were also studied on M. sapientum and M. paradisiaca which are widely used as traditional medicine in Ethiopia, so individuals should be careful of the traditional use of these two species.
ACKNOWLEDGMENTS
First of all, we would like to say thanks to GOD who helps us in every moment of our life with his grace and kindness. Our great appreciation and thanks go to all our colleagues and Addis Ababa University instructors for their valuable comments.
DECLARATION OF INTEREST STATEMENT
We all the authors declare as there is no any conflict of interest.
REFERENCES
- Petrovska BB. (2012). Historical review of medicinal plants’ usage. Pharma Rev. 6(11):1–5.
- Mahomoodally MF. (2013). Evidence Based Complementary and Alternative Medicine Traditional Medicines in Africa : An Appraisal of Ten Potent African Medicinal Plants. Evidence-Based Complementary and Alternative Med. 2013:1-14.
- Hossain M. (2011). Medicinal_Plant_11_Book.Pdf. In the International Union for Conservation of Nature:128.
- Mani S. (2017). Preclinical & Pharmaceutical Research.
- Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A. (2016). Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin Med (United Kingdom). 11(1):1-10.
- Hastuti P, SumardiI, Daryono BS. (2019). Diversity wild banana species (Musa spp.) in Sulawesi, Indonesia. Biodiversitas. 20(3):824-832.
- Moges A, Moges Y. (2020). Ethiopian Common Medicinal Plants: Their Parts and Uses in Traditional Medicine - Ecology and Quality Control. Plant Science - Structure, Anatomy and Physiology in Plants Cultured in Vivo and in Vitro, August.
- Sharrock S. (1998). The banana and its relatives. 52-55.
- Daniells J, Jenny C, Karamura D, Tomekpe,K. (2001). Musalogue: a catalogue of Musa germplasm. Diversity in the genus Musa (E. Arnaud and S. Sharrock, compil.). International Network for the Improvement of Banana and Plantain, Montpellier, France.
- de Vilhena RO, Marson BM, Budel JM, Amano E, Messias-Reason IJ, Pontarolo R. (2019). Morpho-anatomy of the inflorescence of Musa × paradisiaca. Rev Brasileira Farmaco. 29(2):147-151.
- Nayar NM. (2010). The Bananas: Botany, Origin, Dispersal. In Horticultural Reviews.
- Subbaraya U. (2006). Farmers’ knowledge of wild Musa in India. Food and Agriculture Organization of the United Nations, 46.
- Kamira M, Ntamwira J, Sivirihauma C, Ocimati W, van Asten P, Vutseme V, et al. (2016). Agronomic performance of local and introduced plantains, dessert, cooking and beer bananas (Musa spp.) across different altitude and soil conditions in eastern Democratic Republic of Congo. African J Agri Res. 11(43):4313–4332.
- Dixit P, Kumar V, Shukla R. (2014). Therapeutic and medicinal effects of different parts of Musa sapientum. Vivechan Int J Res. 5(1):62-68.
- Galani VJ. (2019). Musa paradisiaca Linn. - A Comprehensive Review. Scholars Int J Trad Complementary Med. 2(4):45-56.
- Sunandar A, Kurniawan AD. (2020). Distribution record of Musa borneensis var. sarawakensis Becc. And Musa campestris var. Sarawakensis Becc. And West Kalimantan, Indonesia. J Trop Biodiversity Biotechnol. 5(3):172–177.
- Pollefeys BP, Sharrock S, Arnaud E. (2004). Preliminary analysis of the literature on the distribution of wild Musa species using MGIS and DIVA-GIS. Inibap. 1-68.
- Häkkinen M, Sharrock S. (2002). Diversity in the genus Musa - Focus on. Institute for the Improvement of Banana and Plantain Annual Report. 2001(1999):16-23.
- Lentfer CJ. (2009). Tracing domestication and cultivation of bananas from phytoliths: An update from Papua New Guinea. Ethnobotany Res Appl. 7: 247-270.
- Hapsari L. (2014). Wild Musa Species Collection of Purwodadi Botanic Garden: Inventory and Its Morpho-taxonomic Review. J Tropical Life Sci. 4(1):70-81.
- Häkkinen M, V̈are H. (2008). A taxonomic revision of Musa aurantiaca (Musaceae) in Southeast Asia. J Syst Evol. 46(1):89-92.
- Kennedy J. (2009). Bananas and people in the homeland of genus Musa: Not just pretty fruit. Ethnobotany Res Appl. 7:179-198.
- Häkkinen M, Väre H. (2008). Taxonomic history and identity of Musa dasycarpa, M. velutina and M. assamica (Musaceae) in Southeast Asia. J Syst Evol. 46(2):230-235.
- Sulistyaningsih LD. (2016). The Diversity of Wild Banana Species (Genus Musa) in Java. Makara J Sci. 20(1).
- Nelson SC, Ploetz RC, Kepler AK. (2006). Musa species (banana and plantain) profiles. Permanent Agriculture Resources. 22:1-28.
- Ajijolakewu KA, Ayoola AS, Agbabiaka TO, Zakariyah FR, Ahmed NR, Oyedele OJ, et al. (2021). A review of the ethnomedicinal, antimicrobial, and phytochemical properties of Musa paradisiaca (plantain). Bull Natl Res Cent. 5(1):86.
- Imam MZ, Akter S. (2011). Musa paradisiacal. and Musa sapientum l. : A phytochemical and pharmacological review. J Appl Pharm Sci. 1(5):14-20.
- Tuasha N, Petros B, Asfaw Z. (2018). Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J Ethnobiol Ethnomed. 14(1):1-21.
- Kidane B, van Andel T, van der Maesen LJG, Asfaw Z. (2014). Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J Ethnobiol Ethnomed. 10(1).
- Oguntibeju OO. (2019). Antidiabetic, Anti-Inflammatory, Antibacterial, Anti-Helminthic, Antioxidant And Nutritional Potential Of Musa paradisiaca. 12(10).
- Kalita H, Boruah DC, Deori M, Hazarika A, Sarma R, Kumari S, et al. (2016). Antidiabetic and antilipidemic effect of Musa balbisiana root extract: A potent agent for glucose homeostasis in streptozotocin-induced diabetic rat. Frontiers Pharmacol. 7:1-11.
- Bandaranayake WM. (1998). Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 2(3):133-148.
- Molina-Salinas GM, Uc-Cachón AH, Peña-Rodríguez LM, Dzul-Beh ADJ, Graća-Medrano RME. (2019). Bactericidal Effect of the Leaf Extract from Musa spp. (AAB Group, Silk Subgroup), cv. Manzano Against Multidrug-Resistant Mycobacterium tuberculosis. J Med Food. 22(11):1183-1185.
- Lacuna-Richman C. (2002). The role of abaca (Musa textilis) in the household economy of a forest village. Small-Scale Forest Economics, Management and Policy. 1(1):93-101.
- Mahadeva Rao US, Mohd KS, Muhammad A, Ahmad BA, Mohamad M, Ali RM. (2014). Taxonomical, phytochemical and pharmacological reviews of Musa sapientum var. Paradisiaca. Res J Pharm Technol. 7(11):1356-1361.
- Ogbonna Obiageli O, Izundu AI, Adachukwu Pauline I, Geraldine Ukamaka O. (2016). Proximate Compositions of fruits of Three Musa Species at Three Stages of Development. J Den Med Sci. 15(6):107-117.
- Oyeyinka BO, Afolayan AJ. (2020). Potentials of Musa Species Fruits against Oxidative Stress-Induced and Diet-Linked Chronic Diseases: In Vitro and In Vivo Implications of Micronutritional Factors and Dietary Secondary Metabolite Compounds. Molecules. 25(21):5036.
- Onyema C, Ofor C, Okudo V, Ogbuagu A. (2016). Phytochemical and Antimicrobial Analysis of Banana Pseudo Stem (Musa acuminata). Br J Pharm Res. 10(1):1-9.
- Oresanya IO, Sonibare MA, Gueye B, Balogun FO, Adebayo S, Ashafa AOT, et al. (2020). Isolation of flavonoids from Musa acuminata Colla (Simili radjah, ABB) and the in vitro inhibitory effects of its leaf and fruit fractions on free radicals, acetylcholinesterase, 15-lipoxygenase, and carbohydrate hydrolyzing enzymes. J Food Biochem. 44(3):1-14.
- Dong C, Hu H, Hu Y, Xie J. (2016). Metabolism of flavonoids in novel banana germplasm during fruit development. Frontiers Plant Sci. 7(AUG2016):1-10.
- Lino PB, Corrěa CF, Archondo MEDL., Dellova DCAL. (2011). Evaluation of post-surgical healing in rats using a topical preparation based on extract of musa sapientum epicarp. Rev Brasileira Farma. 21(3):491-496.
- Jayakumari S, Thiyagarajan R, Devi RS, Loganayaki S, Abinaya AK. (2018). Review on a herbal anticoagulant - Indian Musa species. 10(3):395-399.
- Nofianti T, Muhtadi A, Fidrianny I, Fuadah AS, Nurviana V, Ruswanto. (2021). Klutuk banana (Musa balbisiana colla) peel fractions: Antioxidant and antihyperglycemic potential. Int J Appl Pharma. 13(2):1-6.
- Nascimento REA. (2019). Development of Polymeric Matrices Based on Banana Plant Extracts for Biomedical Applications.
- Sahaa RK, Acharyaa S, Shovon SSH, Royb P. (2013). Medicinal activities of the leaves of Musa sapientum var. Sylvesteris in vitro. Asian Pacific J Trop Biomed. 3(6):476-482.
- Dele S, Eremosele J, Olusola M, Jumoke B. (2019). Nutritional and Phytochemical Assessment of Musa sapientum var. velutina. Pharm Chem J. 6(6):56-64.
- Asuquo EG, Udobi CE. (2016). Antibacterial and toxicity studies of the ethanol extract of Musa paradisiaca leaf . Cogent Biol. 2(1):1219248.
- Hamid HA, Ramli ANM, Yusoff MM. (2017). Indole alkaloids from plants as potential leads for antidepressant drugs: A mini review. Frontiers Pharmacol. 8(2).
- Peel L, Hplc E, Monroy JC, Salem AZM. (2019). Antifungal and Antibacterial Activities of Musa.
- Phillips K, Crews J, Champagne C, Stewart KK. (2011). Contents of issue. J Food Composition Anal. 24(1).
- Islam S. (2013). Phytochemical and Biologcal Investigation of Leaf of Musa sapientum ( Musaceae).
- Mondal A, Banerjee S, Bose S, Das PP, Sandberg EN, Atanasov AG, et al. (2021). Cancer Preventive and Therapeutic Potential of Banana and Its Bioactive Constituents: A Systematic, Comprehensive, and Mechanistic Review. Frontiers in Oncol. 11.
- Liu LC, Lin YH, Lin YC, Ho CT, Hung CM, et al. (2018). Banana flower extract suppresses benign prostatic hyperplasia by regulating the inflammatory response and inducing G1 cell-cycle arrest. In Vivo. 32(6):1373-1379.
- Susanti A, Resti FE, Purbanova R. (2019). Effect of Musa Acuminata Cavendish Subgroup (Ambon Banana) in Reducing Blood Pressure. International Respati Health Conference (IRHC):973-977.
- Jouneghani RS, Castro AHF, Panda SK, Swennen R, Luyten W. (2020). Antimicrobial activity of selected banana cultivars against important human pathogens, including candida biofilm. Foods. 9(4).
- Edition SC, Chemistry A, Aliero T. (2019). A Comparative Phytochemical Analysis And Antimicrobial Activity Of Boswellia dalzielii, ferruginea AND Prosopis africana. 12(1):691-697.
- Chinwe N, Ghasi S, Chukwuemeka AS, NNEKA CM. (2014). Evaluation of the anti-ulcer property of aqueous extract of unripe Musa paradisiacaLinn. peel in Wistar rats. African J Pharm Pharmacol. 8(39):1006-1011.
- Barua N, Das M. (2013). A Review On Pharmacological Activities Of Banana (Musa Paradisiaca). 4(2) 852-858.
- Garcia Mesa MT, Duménigo González A, Acosta de la Luz LL. (2019). Antiallergic potential of a pseudo-stem powder of Musa paradisiacaL. (banana). Int J Phyto Nat Ingred. 6(1):5-5.
- Edenta C, James DB, Owolabi OA, Okoduwa SI. (2014). Hypolipidemic Effects of Aqueous Extract of Three Cultivars of Musa sapientum Fruit Peel on Poloxamer-407 Induced. Int J Pharma Sci Res. 5(12):1046-1051.
- Prasobh GR, Revikumar KG. (2014). A study on the use of musa species in the management of renal calculi. Asian J Pharma Clin Res. 7(3):123–125.
- Sarowar Hossain M, Badrul Alam M, Asadujjaman M, Zahan R, Monirul Islam M, Mazumder MEH, et al. (2011). Antidiarrheal, antioxidant and antimicrobial activities of the musa sapientum seed. Avicenna J Med Biotechnol. 3(2):95-105.
- Cheng YZ, Liu IM, Cheng JT, Lin BS, Liu F. (2020). Wound healing is promoted by Musa paradisiaca(banana) extract in diabetic rats. Arch Med Sci:1-9.
- Mapongmetsem PM, Nkongmeneck BA, Gubbuk H. (2012). The scientific WorldJOURNAL Socioeconomic Importance of the Banana Tree ( Musa Spp .) in the Guinean Highland Savannah Agroforests.
- Wariboko C, Alamene A. (2017). Investigation of some nutritional properties of plantain (Musa paradisiaca) cultivars in Bayelsa State. Eu J Food Sci Technol. 5(3):15-35.
- Edenta C, Okoduwa S, Okpe O. (2017). Effects of Aqueous Extract of Three Cultivars of Banana (Musa acuminata) Fruit Peel on Kidney and Liver Function Indices in Wistar Rats. Med. 4(4):77.
 Abstract
Abstract  PDF
PDF





.PNG)